Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels
- PMID: 35328526
- PMCID: PMC8952255
- DOI: 10.3390/ijms23063105
Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels
Abstract
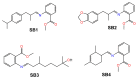
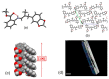
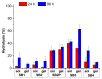
Imines or Schiff bases (SB) are formed by the condensation of an aldehyde or a ketone with a primary amine, with the removal of a water molecule. Schiff bases are central molecules in several biological processes for their ability to form and cleave by small variation of the medium. We report here the controlled hydrolysis of four SBs that may be applied in the fragrance industry, as they are profragrances all containing odorant molecules: methyl anthranilate as primary amine, and four aldehydes (cyclamal, helional, hydroxycitronellal and triplal) that are very volatile odorants. The SB stability was assessed over time by HPLC-MS in neutral or acidic conditions, both in solution and when trapped in low molecular weight gels. Our results demonstrate that it is possible to control the hydrolysis of the Schiff bases in the gel environment, thus tuning the quantity of aldehyde released and the persistency of the fragrance.
Keywords: Schiff base; fragrance; gel; hydrolysis; perfumery; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Design of Stimuli-Responsive Dynamic Covalent Delivery Systems for Volatile Compounds (Part 2): Fragrance-Releasing Cleavable Surfactants in Functional Perfumery Applications.Chemistry. 2021 Sep 20;27(53):13468-13476. doi: 10.1002/chem.202102051. Epub 2021 Aug 6. Chemistry. 2021. PMID: 34270131
-
On Schiff's bases and aldehyde-fuchsin: a review from H. Schiff to R. D. Lillie.Histochemistry. 1981;72(3):321-32. doi: 10.1007/BF00501774. Histochemistry. 1981. PMID: 7028688 Review.
-
Release of Volatile Cyclopentanone Derivatives from Imidazolidin-4-One Profragrances in a Fabric Softener Application.Molecules. 2023 Jan 2;28(1):382. doi: 10.3390/molecules28010382. Molecules. 2023. PMID: 36615574 Free PMC article.
-
Natural Aldehyde-Chitosan Schiff Base: Fabrication, pH-Responsive Properties, and Vegetable Preservation.Foods. 2023 Jul 31;12(15):2921. doi: 10.3390/foods12152921. Foods. 2023. PMID: 37569191 Free PMC article.
-
Schiff bases in medicinal chemistry: a patent review (2010-2015).Expert Opin Ther Pat. 2017 Jan;27(1):63-79. doi: 10.1080/13543776.2017.1252752. Epub 2016 Nov 7. Expert Opin Ther Pat. 2017. PMID: 27774821 Review.
Cited by
-
Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents.Int J Mol Sci. 2023 Feb 28;24(5):4685. doi: 10.3390/ijms24054685. Int J Mol Sci. 2023. PMID: 36902122 Free PMC article. Review.
-
Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels.Molecules. 2023 Mar 10;28(6):2528. doi: 10.3390/molecules28062528. Molecules. 2023. PMID: 36985499 Free PMC article.
-
Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions.Adv Healthc Mater. 2023 Jul;12(17):e2203148. doi: 10.1002/adhm.202203148. Epub 2023 Feb 27. Adv Healthc Mater. 2023. PMID: 36802199 Free PMC article. Review.
-
Controlled Lactonization of o-Coumaric Esters Mediated by Supramolecular Gels.Gels. 2023 Apr 21;9(4):350. doi: 10.3390/gels9040350. Gels. 2023. PMID: 37102962 Free PMC article.
-
Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine.Gels. 2023 Nov 27;9(12):932. doi: 10.3390/gels9120932. Gels. 2023. PMID: 38131918 Free PMC article.
References
-
- Surburg H., Panten J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. 6th ed. Wiley-VCH; Weinheim, Germany: 2016.
-
- Ohloff G., Pickenhagen W., Kraft P. Scent and Chemistry. Wiley-VCH; Weinheim, Germany: 2011.
-
- Berger R.G., editor. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer; Berlin/Heidelberg, Germany: 2007.
-
- Hofmeister I., Landfester K., Taden A. pH-Sensitive Nanocapsules with Barrier Properties: Fragrance Encapsulation and Controlled Release. Macromolecules. 2014;47:5768–5773. doi: 10.1021/ma501388w. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

