Type I-IV Halogen⋯Halogen Interactions: A Comparative Theoretical Study in Halobenzene⋯Halobenzene Homodimers
- PMID: 35328534
- PMCID: PMC8953242
- DOI: 10.3390/ijms23063114
Type I-IV Halogen⋯Halogen Interactions: A Comparative Theoretical Study in Halobenzene⋯Halobenzene Homodimers
Abstract
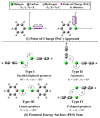
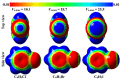
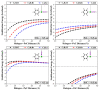
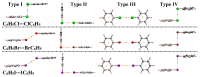
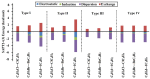
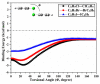
In the current study, unexplored type IV halogen⋯halogen interaction was thoroughly elucidated, for the first time, and compared to the well-established types I−III interactions by means of the second-order Møller−Plesset (MP2) method. For this aim, the halobenzene⋯halobenzene homodimers (where halogen = Cl, Br, and I) were designed into four different types, parodying the considered interactions. From the energetic perspective, the preference of scouted homodimers was ascribed to type II interactions (i.e., highest binding energy), whereas the lowest binding energies were discerned in type III interactions. Generally, binding energies of the studied interactions were observed to decline with the decrease in the σ-hole size in the order, C6H5I⋯IC6H5 > C6H5Br⋯BrC6H5 > C6H5Cl⋯ClC6H5 homodimers and the reverse was noticed in the case of type IV interactions. Such peculiar observations were relevant to the ample contributions of negative-belt⋯negative-belt interactions within the C6H5Cl⋯ClC6H5 homodimer. Further, type IV torsional trans → cis interconversion of C6H5X⋯XC6H5 homodimers was investigated to quantify the π⋯π contributions into the total binding energies. Evidently, the energetic features illustrated the amelioration of the considered homodimers (i.e., more negative binding energy) along the prolonged scope of torsional trans → cis interconversion. In turn, these findings outlined the efficiency of the cis configuration over the trans analog. Generally, symmetry-adapted perturbation theory-based energy decomposition analysis (SAPT-EDA) demonstrated the predominance of all the scouted homodimers by the dispersion forces. The obtained results would be beneficial for the omnipresent studies relevant to the applications of halogen bonds in the fields of materials science and crystal engineering.
Keywords: QTAIM; SAPT-EDA; halogen bond; trans → cis interconversion; σ-hole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

