Kappa-Opioid Receptor Blockade Ameliorates Obesity Caused by Estrogen Withdrawal via Promotion of Energy Expenditure through mTOR Pathway
- PMID: 35328539
- PMCID: PMC8953356
- DOI: 10.3390/ijms23063118
Kappa-Opioid Receptor Blockade Ameliorates Obesity Caused by Estrogen Withdrawal via Promotion of Energy Expenditure through mTOR Pathway
Abstract
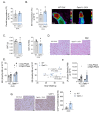
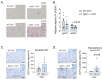
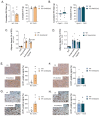
Weight gain is a hallmark of decreased estradiol (E2) levels because of menopause or following surgical ovariectomy (OVX) at younger ages. Of note, this weight gain tends to be around the abdomen, which is frequently associated with impaired metabolic homeostasis and greater cardiovascular risk in both rodents and humans. However, the molecular underpinnings and the neuronal basis for these effects remain to be elucidated. The aim of this study is to elucidate whether the kappa-opioid receptor (k-OR) system is involved in mediating body weight changes associated with E2 withdrawal. Here, we document that body weight gain induced by OVX occurs, at least partially, in a k-OR dependent manner, by modulation of energy expenditure independently of food intake as assessed in Oprk1-/-global KO mice. These effects were also observed following central pharmacological blockade of the k-OR system using the k-OR-selective antagonist PF-04455242 in wild type mice, in which we also observed a decrease in OVX-induced weight gain associated with increased UCP1 positive immunostaining in brown adipose tissue (BAT) and browning of white adipose tissue (WAT). Remarkably, the hypothalamic mTOR pathway plays an important role in regulating weight gain and adiposity in OVX mice. These findings will help to define new therapies to manage metabolic disorders associated with low/null E2 levels based on the modulation of central k-OR signaling.
Keywords: energy expenditure; estrogens; kappa-opioid; obesity; p70S6K.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
A Combination of a Dopamine Receptor 2 Agonist and a Kappa Opioid Receptor Antagonist Synergistically Reduces Weight in Diet-Induced Obese Rodents.Nutrients. 2024 Jan 31;16(3):424. doi: 10.3390/nu16030424. Nutrients. 2024. PMID: 38337707 Free PMC article.
-
Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen.Biochem Biophys Res Commun. 2001 Oct 19;288(1):191-7. doi: 10.1006/bbrc.2001.5763. Biochem Biophys Res Commun. 2001. PMID: 11594772
-
Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction.Am J Physiol Regul Integr Comp Physiol. 2015 Mar 15;308(6):R530-42. doi: 10.1152/ajpregu.00401.2014. Epub 2015 Jan 21. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25608751 Free PMC article.
-
Estradiol Regulation of Brown Adipose Tissue Thermogenesis.Adv Exp Med Biol. 2017;1043:315-335. doi: 10.1007/978-3-319-70178-3_15. Adv Exp Med Biol. 2017. PMID: 29224101 Review.
-
Estradiol effects on hypothalamic AMPK and BAT thermogenesis: A gateway for obesity treatment?Pharmacol Ther. 2017 Oct;178:109-122. doi: 10.1016/j.pharmthera.2017.03.014. Epub 2017 Mar 27. Pharmacol Ther. 2017. PMID: 28351720 Review.
Cited by
-
A Combination of a Dopamine Receptor 2 Agonist and a Kappa Opioid Receptor Antagonist Synergistically Reduces Weight in Diet-Induced Obese Rodents.Nutrients. 2024 Jan 31;16(3):424. doi: 10.3390/nu16030424. Nutrients. 2024. PMID: 38337707 Free PMC article.
-
Adipocyte Metabolism and Health after the Menopause: The Role of Exercise.Nutrients. 2023 Jan 14;15(2):444. doi: 10.3390/nu15020444. Nutrients. 2023. PMID: 36678314 Free PMC article. Review.
-
Hypothalamic Estrogen Signaling and Adipose Tissue Metabolism in Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 9;13:898139. doi: 10.3389/fendo.2022.898139. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35757435 Free PMC article. Review.
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
Molecular mechanisms underlying glucose-dependent insulinotropic polypeptide secretion in human duodenal organoids.Diabetologia. 2025 Jan;68(1):217-230. doi: 10.1007/s00125-024-06293-3. Epub 2024 Oct 23. Diabetologia. 2025. PMID: 39441374 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

