Structural Modifications on Chalcone Framework for Developing New Class of Cholinesterase Inhibitors
- PMID: 35328542
- PMCID: PMC8953944
- DOI: 10.3390/ijms23063121
Structural Modifications on Chalcone Framework for Developing New Class of Cholinesterase Inhibitors
Abstract
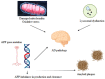
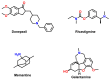
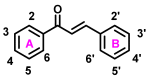
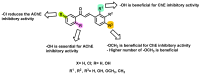
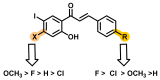
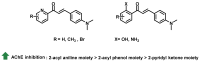
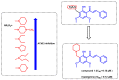
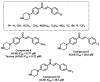
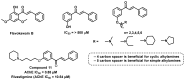
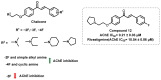
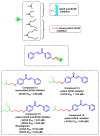
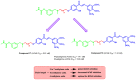
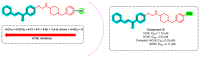
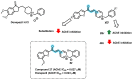
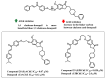
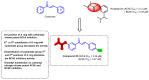
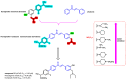
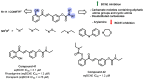
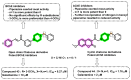
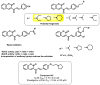
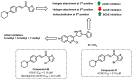
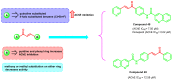
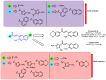
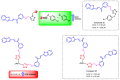
Due to the multifaceted pharmacological activities of chalcones, these scaffolds have been considered one of the most privileged frameworks in the drug discovery process. Structurally, chalcones are α, β-unsaturated carbonyl functionalities with two aryl or heteroaryl units. Amongst the numerous pharmacological activities explored for chalcone derivatives, the development of novel chalcone analogs for the treatment of Alzheimer's disease (AD) is among the research topics of most interest. Chalcones possess numerous advantages, such as smaller molecular size, opportunities for further structural modification thereby altering the physicochemical properties, cost-effectiveness, and convenient synthetic methodology. The present review highlights the recent evidence of chalcones as a privileged structure in AD drug development processes. Different classes of chalcone-derived analogs are summarized for the easy understanding of the previously reported analogs as well as the importance of certain functionalities in exhibiting cholinesterase inhibition. In this way, this review will shed light on the medicinal chemistry fraternity for the design and development of novel promising chalcone candidates for the treatment of AD.
Keywords: acetylcholinesterase; butyrylcholinesterase; chalcones; structure activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
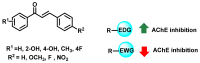
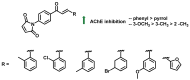
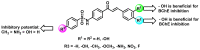
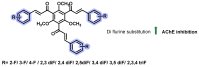

Similar articles
-
Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer's disease.Bioorg Chem. 2021 Mar;108:104681. doi: 10.1016/j.bioorg.2021.104681. Epub 2021 Jan 29. Bioorg Chem. 2021. PMID: 33571811 Free PMC article. Review.
-
Small Multitarget Molecules Incorporating the Enone Moiety.Molecules. 2019 Jan 7;24(1):199. doi: 10.3390/molecules24010199. Molecules. 2019. PMID: 30621100 Free PMC article.
-
Cholinesterases inhibition and molecular modeling studies of piperidyl-thienyl and 2-pyrazoline derivatives of chalcones.Biochem Biophys Res Commun. 2017 Jan 22;482(4):615-624. doi: 10.1016/j.bbrc.2016.11.082. Epub 2016 Nov 16. Biochem Biophys Res Commun. 2017. PMID: 27865835
-
Structure-activity relationship investigation of coumarin-chalcone hybrids with diverse side-chains as acetylcholinesterase and butyrylcholinesterase inhibitors.Mol Divers. 2018 Nov;22(4):893-906. doi: 10.1007/s11030-018-9839-y. Epub 2018 Jun 22. Mol Divers. 2018. PMID: 29934672 Free PMC article.
-
Privileged Pharmacophore of FDA Approved Drugs in Combination with Chalcone Framework: A New Hope for Alzheimer's Treatment.Comb Chem High Throughput Screen. 2020;23(9):842-846. doi: 10.2174/1386207323999200728122627. Comb Chem High Throughput Screen. 2020. PMID: 32723232 Review.
Cited by
-
Ultrasound-Assisted Synthesis of 2‑Benzylidene-1-Indanone Derivatives and Evaluation as a Corrosion Inhibitor for Mild Steel in 1 M HCl Solution.ACS Omega. 2025 May 20;10(21):21147-21161. doi: 10.1021/acsomega.4c09705. eCollection 2025 Jun 3. ACS Omega. 2025. PMID: 40488013 Free PMC article.
-
Bacopa monnieri: A promising herbal approach for neurodegenerative disease treatment supported by in silico and in vitro research.Heliyon. 2023 Oct 20;9(11):e21161. doi: 10.1016/j.heliyon.2023.e21161. eCollection 2023 Nov. Heliyon. 2023. PMID: 37954293 Free PMC article.
-
Unveiling potent inhibitors for schistosomiasis through ligand-based drug design, molecular docking, molecular dynamics simulations and pharmacokinetics predictions.PLoS One. 2024 Jun 26;19(6):e0302390. doi: 10.1371/journal.pone.0302390. eCollection 2024. PLoS One. 2024. PMID: 38923997 Free PMC article.
-
Insights on the Anti-Inflammatory and Anti-Melanogenic Effects of 2'-Hydroxy-2,6'-dimethoxychalcone in RAW 264.7 and B16F10 Cells.Curr Issues Mol Biol. 2025 Jan 29;47(2):85. doi: 10.3390/cimb47020085. Curr Issues Mol Biol. 2025. PMID: 39996806 Free PMC article.
-
Chalcone Scaffolds Exhibiting Acetylcholinesterase Enzyme Inhibition: Mechanistic and Computational Investigations.Molecules. 2022 May 16;27(10):3181. doi: 10.3390/molecules27103181. Molecules. 2022. PMID: 35630658 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

