Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents
- PMID: 35328544
- PMCID: PMC8950963
- DOI: 10.3390/ijms23063123
Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents
Abstract
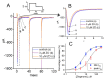
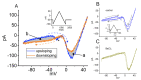
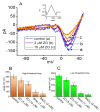
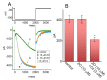
Zingerone (ZO), a nontoxic methoxyphenol, has been demonstrated to exert various important biological effects. However, its action on varying types of ionic currents and how they concert in neuronal cells remain incompletely understood. With the aid of patch clamp technology, we investigated the effects of ZO on the amplitude, gating, and hysteresis of plasmalemmal ionic currents from both pituitary tumor (GH3) cells and hippocampal (mHippoE-14) neurons. The exposure of the GH3 cells to ZO differentially diminished the peak and late components of the INa. Using a double ramp pulse, the amplitude of the INa(P) was measured, and the appearance of a hysteresis loop was observed. Moreover, ZO reversed the tefluthrin-mediated augmentation of the hysteretic strength of the INa(P) and led to a reduction in the ICa,L. As a double ramp pulse was applied, two types of voltage-dependent hysteresis loops were identified in the ICa,L, and the replacement with BaCl2-attenuated hysteresis of the ICa,L enhanced the ICa,L amplitude along with the current amplitude (i.e., the IBa). The hysteretic magnitude of the ICa,L activated by the double pulse was attenuated by ZO. The peak and late INa in the hippocampal mHippoE-14 neurons was also differentially inhibited by ZO. In addition to acting on the production of reactive oxygen species, ZO produced effects on multiple ionic currents demonstrated herein that, considered together, may significantly impact the functional activities of neuronal cells.
Keywords: L-type Ca2+ current; hysteresis; persistent Na+ current; vanillylacetone); voltage-gated Na+ current; zingerone (gingerone.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4'-Hydroxy-3'-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor.Biomedicines. 2021 Sep 3;9(9):1146. doi: 10.3390/biomedicines9091146. Biomedicines. 2021. PMID: 34572332 Free PMC article.
-
The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current.Nutrients. 2022 Aug 18;14(16):3393. doi: 10.3390/nu14163393. Nutrients. 2022. PMID: 36014898 Free PMC article.
-
Effectiveness of Columbianadin, a Bioactive Coumarin Derivative, in Perturbing Transient and Persistent INa.Int J Mol Sci. 2021 Jan 9;22(2):621. doi: 10.3390/ijms22020621. Int J Mol Sci. 2021. PMID: 33435511 Free PMC article.
-
Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents.Int J Mol Sci. 2022 Aug 21;23(16):9453. doi: 10.3390/ijms23169453. Int J Mol Sci. 2022. PMID: 36012718 Free PMC article. Review.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
Cited by
-
Immunity, Ion Channels and Epilepsy.Int J Mol Sci. 2022 Jun 9;23(12):6446. doi: 10.3390/ijms23126446. Int J Mol Sci. 2022. PMID: 35742889 Free PMC article. Review.
-
Anesthetic- and Analgesic-Related Drugs Modulating Both Voltage-Gated Na+ and TRP Channels.Biomolecules. 2024 Dec 18;14(12):1619. doi: 10.3390/biom14121619. Biomolecules. 2024. PMID: 39766326 Free PMC article. Review.
-
Zingerone alleviates inflammatory pain by reducing the intrinsic excitability of anterior cingulate cortex neurons in a mice model.Front Pharmacol. 2025 Mar 11;16:1543594. doi: 10.3389/fphar.2025.1543594. eCollection 2025. Front Pharmacol. 2025. PMID: 40135239 Free PMC article.
-
Rufinamide, a Triazole-Derived Antiepileptic Drug, Stimulates Ca2+-Activated K+ Currents While Inhibiting Voltage-Gated Na+ Currents.Int J Mol Sci. 2022 Nov 8;23(22):13677. doi: 10.3390/ijms232213677. Int J Mol Sci. 2022. PMID: 36430153 Free PMC article.
-
Concerted suppressive effects of carisbamate, an anti-epileptic alkyl-carbamate drug, on voltage-gated Na+ and hyperpolarization-activated cation currents.Front Cell Neurosci. 2023 May 24;17:1159067. doi: 10.3389/fncel.2023.1159067. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37293624 Free PMC article.
References
-
- Alsherbiny M.A., Abd-Elsalam W.H., El-Badawy S.A., Taher E., Fares M., Torres A., Chang D., Li C.G. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2019;123:72–97. doi: 10.1016/j.fct.2018.10.048. - DOI - PubMed
-
- Rashid S., Wali A.F., Rashid S.M., Alsaffar R.M., Ahmad A., Jan B.L., Paray B.A., Alqahtani S.M.A., Arafah A., Rehman M.U. Zingerone targets status epilepticus by blocking hippocampal neurodegeneration via regulation of redox imbalance, inflammation and apoptosis. Pharmaceuticals. 2021;14:146. doi: 10.3390/ph14020146. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

