Signal Sequence-Dependent Orientation of Signal Peptide Fragments to Exosomes
- PMID: 35328557
- PMCID: PMC8950404
- DOI: 10.3390/ijms23063137
Signal Sequence-Dependent Orientation of Signal Peptide Fragments to Exosomes
Abstract
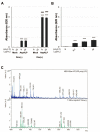
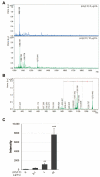
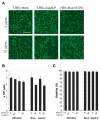
Signal peptides (SPs) not only mediate targeting to the endoplasmic reticulum (ER) but also play important roles as biomarkers and substances with physiological activity in extracellular fluids including blood. SPs are thought to be degraded intracellularly, making it unclear how they are transported from the ER to the extracellular fluid. In a recent study, we showed that a C-terminal fragment of the SP of a type I membrane protein, amyloid precursor protein (APP), was secreted into the extracellular fluid via exosomes using transformed HEK293 cells expressing APP SP flanking a reporter protein. In the present study, we demonstrate that a N-terminal fragment of the SP from a type II membrane protein, human placental secreted alkaline phosphatase (SEAP), is contained in exosomes and secreted into the extracellular fluid using HEK-Blue hTLR3 cells, which express both a human toll-like receptor 3 gene and an inducible SEAP reporter gene. When HEK-Blue hTLR3 cells were stimulated with a TLR3 ligand, a N-terminal fragment of SEAP SP in exosomes was increased in parallel with SEAP secretion in a concentration-dependent manner. These results indicated that SP fragments are exosomal components. In addition, migrating SP fragments were determined by characteristics of the signal-anchor sequence of membrane proteins. Furthermore, we found that SP fragments could bind to calmodulin (CALM), which is a cytosolic protein and also a component of exosomes, suggesting its involvement in the transportation of SP fragments from the endoplasmic reticulum to exosomes.
Keywords: calmodulin; exosomes; intercellular communication; signal peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pemberton C.J., Siriwardena M., Kleffmann T., Ruygrok P., Palmer S.C., Yandle T.G., Richards A.M. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: Initial assessment as cardiovascular biomarkers. Clin. Chem. 2012;58:757–767. doi: 10.1373/clinchem.2011.176990. - DOI - PubMed
-
- Siriwardena M., Kleffmann T., Ruygrok P., Cameron V.A., Yandle T.G., Nicholls M.G., Richards A.M., Pemberton C.J. B-type natriuretic peptide signal peptide circulates in human blood: Evaluation as a potential biomarker of cardiac ischemia. Circulation. 2010;122:255–264. doi: 10.1161/CIRCULATIONAHA.109.909937. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

