Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3'-UTR of the CHRNA 6 Gene
- PMID: 35328565
- PMCID: PMC8948824
- DOI: 10.3390/ijms23063145
Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3'-UTR of the CHRNA 6 Gene
Abstract
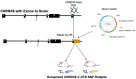
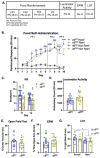
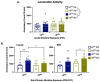
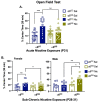
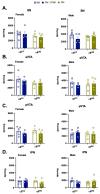
In human adolescents, a single nucleotide polymorphism (SNP), rs2304297, in the 3'-UTR of the nicotinic receptor subunit gene, CHRNA6, has been associated with increased smoking. To study the effects of the human CHRNA6 3'-UTR SNP, our lab generated knock-in rodent lines with either C or G SNP alleles. The objective of this study was to determine if the CHRNA6 3'-UTR SNP is functional in the knock-in rat lines. We hypothesized that the human CHRNA6 3'-UTR SNP knock-in does not impact baseline but enhances nicotine-induced behaviors. For baseline behaviors, rats underwent food self-administration at escalating schedules of reinforcement followed by a locomotor assay and a series of anxiety tests (postnatal day (PN) 25-39). In separate cohorts, adolescent rats underwent 1- or 4-day nicotine pretreatment (2×, 30 μg/kg/0.1 mL, i.v.). After the last nicotine injection (PN 31), animals were assessed behaviorally in an open-field chamber, and brain tissue was collected. We show the human CHRNA6 3'-UTR SNP knock-in does not affect food reinforcement, locomotor activity, or anxiety. Further, 4-day, but not 1-day, nicotine exposure enhances locomotion and anxiolytic behavior in a genotype- and sex-specific manner. These findings demonstrate that the human CHRNA6 3'-UTR SNP is functional in our in vivo model.
Keywords: CHRNA6; adolescent; anxiety-like behavior; locomotor activity; pharmacogenetics.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. US Department of Health and Human Services; Washington, DC, USA: 2014.
-
- NIDA “What Is the Scope of Tobacco, Nicotine, and e-Cigarette Use in the United States?” National Institute on Drug Abuse. [(accessed on 13 March 2022)];2022 February 8; Available online: https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-ci....
MeSH terms
Substances
Grants and funding
- 22RT-0103/Tobacco-Related Disease Research Program
- 21517/Brain and Behavior Research Grant
- R01, DA048899/NH/NIH HHS/United States
- UL1 TR001414 (SL)/University of California, Irvine (UCI) Institute for Clinical and Translational Sciences (ICTS) Pilot Studies Program (National Institute of Health, National Center for Advancing Translational Science (NIH, NCATS))
LinkOut - more resources
Full Text Sources
Miscellaneous

