cADPR Does Not Activate TRPM2
- PMID: 35328585
- PMCID: PMC8949931
- DOI: 10.3390/ijms23063163
cADPR Does Not Activate TRPM2
Abstract
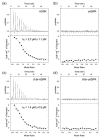
cADPR is a second messenger that releases Ca2+ from intracellular stores via the ryanodine receptor. Over more than 15 years, it has been controversially discussed whether cADPR also contributes to the activation of the nucleotide-gated cation channel TRPM2. While some groups have observed activation of TRPM2 by cADPR alone or in synergy with ADPR, sometimes only at 37 °C, others have argued that this is due to the contamination of cADPR by ADPR. The identification of a novel nucleotide-binding site in the N-terminus of TRPM2 that binds ADPR in a horseshoe-like conformation resembling cADPR as well as the cADPR antagonist 8-Br-cADPR, and another report that demonstrates activation of TRPM2 by binding of cADPR to the NUDT9H domain raised the question again and led us to revisit the topic. Here we show that (i) the N-terminal MHR1/2 domain and the C-terminal NUDT9H domain are required for activation of human TRPM2 by ADPR and 2'-deoxy-ADPR (2dADPR), (ii) that pure cADPR does not activate TRPM2 under a variety of conditions that have previously been shown to result in channel activation, (iii) the cADPR antagonist 8-Br-cADPR also inhibits activation of TRPM2 by ADPR, and (iv) cADPR does not bind to the MHR1/2 domain of TRPM2 while ADPR does.
Keywords: calcium signalling; cyclic adenosine 5′-diphosphate ribose; second messenger; transient receptor potential channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lee H.C. The Cyclic ADP-Ribose/NAADP/CD38-Signaling Pathway: Past and Present. Messenger. 2012;1:16–33. doi: 10.1166/msr.2012.1005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

