Effects of a Fully Humanized Type II Anti-CD20 Monoclonal Antibody on Peripheral and CNS B Cells in a Transgenic Mouse Model of Multiple Sclerosis
- PMID: 35328594
- PMCID: PMC8949956
- DOI: 10.3390/ijms23063172
Effects of a Fully Humanized Type II Anti-CD20 Monoclonal Antibody on Peripheral and CNS B Cells in a Transgenic Mouse Model of Multiple Sclerosis
Abstract
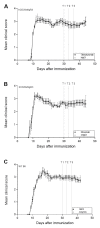
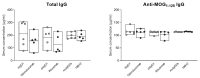
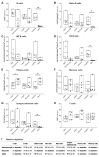
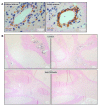
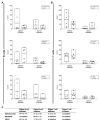
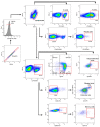
Successful therapy with anti-CD20 monoclonal antibodies (mAbs) has reinforced the key role of B cells in the immunopathology of multiple sclerosis (MS). This study aimed to determine the effects of a novel class of anti-CD20 mAbs on vascular and extravascular central nervous system (CNS)-infiltrating B cells in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Male hCD20xhIgR3 mice and wild-type C57BL/6 (B6) mice were immunized with human myelin oligodendrocyte glycoprotein (MOG)1-125 to induce EAE. While hCD20xhIgR3 mice were injected intravenously with an anti-human CD20 mAb (5 mg/kg) (rituximab (a type I anti-CD20 mAb) or obinutuzumab (a type II anti-CD20 mAb), B6 mice received the anti-mouse CD20 antibody 18B12. Neither mAb affected clinical disease or serum antibody levels. Obinutuzumab and rituximab had an impact on splenic and CNS-infiltrated B cells with slightly differential depletion efficacy. Additionally, obinutuzumab had beneficial effects on spinal cord myelination. B cell depletion rates in the 18B12/B6 model were comparable with those observed in obinutuzumab-treated hCD20xhIgR3 mice. Our results demonstrate the usefulness of anti-CD20 mAbs for the modulation of B cell-driven peripheral immune response and CNS pathology, with type II antibodies potentially being superior to type I in the depletion of tissue-infiltrating B cells.
Keywords: B cells; CD20; experimental autoimmune encephalomyelitis; multiple sclerosis; obinutuzumab; rituximab.
Conflict of interest statement
S.T., R.C. and V.S. declare that they have no competing interests. E.U. is an employee of F. Hoffmann-La Roche. S.K. reports grants from the Deutsche Forschungsgemeinschaft (DFG), Novartis, F. Hoffmann-La Roche, and Sanofi; and speaker fees and consultancy honoraria from Novartis, F. Hoffmann-La Roche, Sanofi, and Teva (outside the submitted work). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Obinutuzumab-Induced B Cell Depletion Reduces Spinal Cord Pathology in a CD20 Double Transgenic Mouse Model of Multiple Sclerosis.Int J Mol Sci. 2020 Sep 18;21(18):6864. doi: 10.3390/ijms21186864. Int J Mol Sci. 2020. PMID: 32962135 Free PMC article.
-
Efficient Distribution of a Novel Zirconium-89 Labeled Anti-cd20 Antibody Following Subcutaneous and Intravenous Administration in Control and Experimental Autoimmune Encephalomyelitis-Variant Mice.Front Immunol. 2019 Oct 18;10:2437. doi: 10.3389/fimmu.2019.02437. eCollection 2019. Front Immunol. 2019. PMID: 31681317 Free PMC article.
-
CAR-T Cell-Mediated B-Cell Depletion in Central Nervous System Autoimmunity.Neurol Neuroimmunol Neuroinflamm. 2023 Jan 19;10(2):e200080. doi: 10.1212/NXI.0000000000200080. Print 2023 Mar. Neurol Neuroimmunol Neuroinflamm. 2023. PMID: 36657993 Free PMC article.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
A Milestone in Multiple Sclerosis Therapy: Monoclonal Antibodies Against CD20-Yet Progress Continues.Neurotherapeutics. 2021 Jul;18(3):1602-1622. doi: 10.1007/s13311-021-01048-z. Epub 2021 Apr 20. Neurotherapeutics. 2021. PMID: 33880738 Free PMC article. Review.
Cited by
-
Preclinical B cell depletion and safety profile of a brain-shuttled crystallizable fragment-silenced CD20 antibody.Clin Transl Med. 2025 Mar;15(3):e70178. doi: 10.1002/ctm2.70178. Clin Transl Med. 2025. PMID: 40118783 Free PMC article.
-
The presence of cerebellar B cell aggregates is associated with a specific chemokine profile in the cerebrospinal fluid in a mouse model of multiple sclerosis.J Neuroinflammation. 2023 Jan 30;20(1):18. doi: 10.1186/s12974-023-02695-z. J Neuroinflammation. 2023. PMID: 36717913 Free PMC article.
-
CD19+ B cell depletion: a novel strategy to alleviate ischemic stroke damage.Front Immunol. 2025 Apr 17;16:1528471. doi: 10.3389/fimmu.2025.1528471. eCollection 2025. Front Immunol. 2025. PMID: 40313936 Free PMC article.
-
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function.CNS Drugs. 2025 Jun;39(6):545-564. doi: 10.1007/s40263-025-01182-8. Epub 2025 Apr 3. CNS Drugs. 2025. PMID: 40180777 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

