BRET-Based Biosensors to Measure Agonist Efficacies in Histamine H1 Receptor-Mediated G Protein Activation, Signaling and Interactions with GRKs and β-Arrestins
- PMID: 35328605
- PMCID: PMC8953162
- DOI: 10.3390/ijms23063184
BRET-Based Biosensors to Measure Agonist Efficacies in Histamine H1 Receptor-Mediated G Protein Activation, Signaling and Interactions with GRKs and β-Arrestins
Abstract
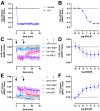
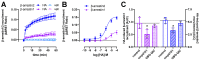
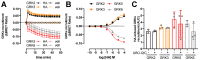
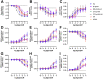
The histamine H1 receptor (H1R) is a G protein-coupled receptor (GPCR) and plays a key role in allergic reactions upon activation by histamine which is locally released from mast cells and basophils. Consequently, H1R is a well-established therapeutic target for antihistamines that relieve allergy symptoms. H1R signals via heterotrimeric Gq proteins and is phosphorylated by GPCR kinase (GRK) subtypes 2, 5, and 6, consequently facilitating the subsequent recruitment of β-arrestin1 and/or 2. Stimulation of a GPCR with structurally different agonists can result in preferential engagement of one or more of these intracellular signaling molecules. To evaluate this so-called biased agonism for H1R, bioluminescence resonance energy transfer (BRET)-based biosensors were applied to measure H1R signaling through heterotrimeric Gq proteins, second messengers (inositol 1,4,5-triphosphate and Ca2+), and receptor-protein interactions (GRKs and β-arrestins) in response to histamine, 2-phenylhistamines, and histaprodifens in a similar cellular background. Although differences in efficacy were observed for these agonists between some functional readouts as compared to reference agonist histamine, subsequent data analysis using an operational model of agonism revealed only signaling bias of the agonist Br-phHA-HA in recruiting β-arrestin2 to H1R over Gq biosensor activation.
Keywords: BRET assay; G protein; GPCR; GPCR kinase; H1R; biased signaling; histamine; β-arrestin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Histamine H1 Receptor-Mediated JNK Phosphorylation Is Regulated by Gq Protein-Dependent but Arrestin-Independent Pathways.Int J Mol Sci. 2024 Mar 17;25(6):3395. doi: 10.3390/ijms25063395. Int J Mol Sci. 2024. PMID: 38542369 Free PMC article.
-
Label-free versus conventional cellular assays: Functional investigations on the human histamine H1 receptor.Pharmacol Res. 2016 Dec;114:13-26. doi: 10.1016/j.phrs.2016.10.010. Epub 2016 Oct 15. Pharmacol Res. 2016. PMID: 27751876
-
G protein-coupled receptor kinases (GRKs) orchestrate biased agonism at the β2-adrenergic receptor.Sci Signal. 2018 Aug 21;11(544):eaar7084. doi: 10.1126/scisignal.aar7084. Sci Signal. 2018. PMID: 30131371
-
G protein-coupled receptor interactions with arrestins and GPCR kinases: The unresolved issue of signal bias.J Biol Chem. 2022 Sep;298(9):102279. doi: 10.1016/j.jbc.2022.102279. Epub 2022 Jul 19. J Biol Chem. 2022. PMID: 35863432 Free PMC article. Review.
-
Phosphorylation barcoding as a mechanism of directing GPCR signaling.Sci Signal. 2011 Aug 9;4(185):pe36. doi: 10.1126/scisignal.2002331. Sci Signal. 2011. PMID: 21868354 Review.
Cited by
-
Interaction between Nanoparticles, Membranes and Proteins: A Surface Plasmon Resonance Study.Int J Mol Sci. 2022 Dec 29;24(1):591. doi: 10.3390/ijms24010591. Int J Mol Sci. 2022. PMID: 36614033 Free PMC article.
-
Exploring GPR109A Receptor Interaction with Hippuric Acid Using MD Simulations and CD Spectroscopy.Int J Mol Sci. 2022 Nov 26;23(23):14778. doi: 10.3390/ijms232314778. Int J Mol Sci. 2022. PMID: 36499106 Free PMC article.
-
Optimization of Biotinylated RNA or DNA Pull-Down Assays for Detection of Binding Proteins: Examples of IRP1, IRP2, HuR, AUF1, and Nrf2.Int J Mol Sci. 2023 Feb 10;24(4):3604. doi: 10.3390/ijms24043604. Int J Mol Sci. 2023. PMID: 36835018 Free PMC article.
-
Detecting and measuring of GPCR signaling - comparison of human induced pluripotent stem cells and immortal cell lines.Front Endocrinol (Lausanne). 2023 May 24;14:1179600. doi: 10.3389/fendo.2023.1179600. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37293485 Free PMC article. Review.
-
Receptor-Ligand Binding: Effect of Mechanical Factors.Int J Mol Sci. 2023 May 21;24(10):9062. doi: 10.3390/ijms24109062. Int J Mol Sci. 2023. PMID: 37240408 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

