Structural Plasticity Is a Feature of Rheostat Positions in the Human Na+/Taurocholate Cotransporting Polypeptide (NTCP)
- PMID: 35328632
- PMCID: PMC8954283
- DOI: 10.3390/ijms23063211
Structural Plasticity Is a Feature of Rheostat Positions in the Human Na+/Taurocholate Cotransporting Polypeptide (NTCP)
Abstract
In the Na+/taurocholate cotransporting polypeptide (NTCP), the clinically relevant S267F polymorphism occurs at a "rheostat position". That is, amino acid substitutions at this position ("S267X") lead to a wide range of functional outcomes. This result was particularly striking because molecular models predicted the S267X side chains are buried, and thus, usually expected to be less tolerant of substitutions. To assess whether structural tolerance to buried substitutions is widespread in NTCP, here we used Rosetta to model all 19 potential substitutions at another 13 buried positions. Again, only subtle changes in the calculated stabilities and structures were predicted. Calculations were experimentally validated for 19 variants at codon 271 ("N271X"). Results showed near wildtype expression and rheostatic modulation of substrate transport, implicating N271 as a rheostat position. Notably, each N271X substitution showed a similar effect on the transport of three different substrates and thus did not alter substrate specificity. This differs from S267X, which altered both transport kinetics and specificity. As both transport and specificity may change during protein evolution, the recognition of such rheostat positions may be important for evolutionary studies. We further propose that the presence of rheostat positions is facilitated by local plasticity within the protein structure. Finally, we note that identifying rheostat positions may advance efforts to predict new biomedically relevant missense variants in NTCP and other membrane transport proteins.
Keywords: protein plasticity; rheostat; transmembrane protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

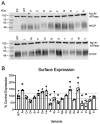
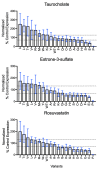


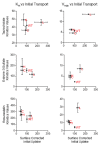
References
-
- Pan W., Song I.S., Shin H.J., Kim M.H., Choi Y.L., Lim S.J., Kim W.Y., Lee S.S., Shin J.G. Genetic polymorphisms in Na+-taurocholate co-transporting polypeptide (NTCP) and ileal apical sodium-dependent bile acid transporter (ASBT) and ethnic comparisons of functional variants of NTCP among Asian populations. Xenobiotica. 2011;41:501–510. doi: 10.3109/00498254.2011.555567. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

