4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents
- PMID: 35328634
- PMCID: PMC8955734
- DOI: 10.3390/ijms23063213
4-Arylthiosemicarbazide Derivatives as Toxoplasmic Aromatic Amino Acid Hydroxylase Inhibitors and Anti-inflammatory Agents
Abstract
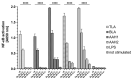
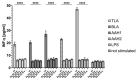
Approximately one-third of the human population is infected with the intracellular cosmopolitan protozoan Toxoplasma gondii (Tg), and a specific treatment for this parasite is still needed. Additionally, the increasing resistance of Tg to drugs has become a challenge for numerous research centers. The high selectivity of a compound toward the protozoan, along with low cytotoxicity toward the host cells, form the basis for further research, which aims at determining the molecular targets of the active compounds. Thiosemicarbazide derivatives are biologically active organic compounds. Previous studies on the initial preselection of 58 new 4-arylthiosemicarbazide derivatives in terms of their anti-Tg activity and selectivity made it possible to select two promising derivatives for further research. One of the important amino acids involved in the proliferation of Tg and the formation of parasitophorous vacuoles is tyrosine, which is converted by two unique aromatic amino acid hydroxylases to levodopa. Enzymatic studies with two derivatives (R: para-nitro and meta-iodo) and recombinant aromatic amino acid hydroxylase (AAHs) obtained in the E. coli expression system were performed, and the results indicated that toxoplasmic AAHs are a molecular target for 4-arylthiosemicarbazide derivatives. Moreover, the drug affinity responsive target stability assay also confirmed that the selected compounds bind to AAHs. Additionally, the anti-inflammatory activity of these derivatives was tested using THP1-Blue™ NF-κB reporter cells due to the similarity of the thiosemicarbazide scaffold to thiosemicarbazone, both of which are known NF-κB pathway inhibitors.
Keywords: NF-κB pathway inhibition; Toxoplasma gondii; bradyzoite; thiosemicarbazide; tyrosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
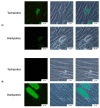

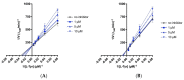


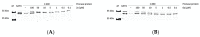
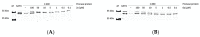
References
-
- Checkley W., White A.C., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015;15:85–94. doi: 10.1016/S1473-3099(14)70772-8. - DOI - PMC - PubMed
-
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

