Characterization of a Novel Aspect of Tissue Scarring Following Experimental Spinal Cord Injury and the Implantation of Bioengineered Type-I Collagen Scaffolds in the Adult Rat: Involvement of Perineurial-like Cells?
- PMID: 35328642
- PMCID: PMC8954100
- DOI: 10.3390/ijms23063221
Characterization of a Novel Aspect of Tissue Scarring Following Experimental Spinal Cord Injury and the Implantation of Bioengineered Type-I Collagen Scaffolds in the Adult Rat: Involvement of Perineurial-like Cells?
Abstract
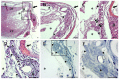
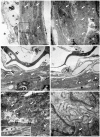
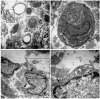
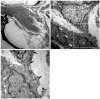
Numerous intervention strategies have been developed to promote functional tissue repair following experimental spinal cord injury (SCI), including the bridging of lesion-induced cystic cavities with bioengineered scaffolds. Integration between such implanted scaffolds and the lesioned host spinal cord is critical for supporting regenerative growth, but only moderate-to-low degrees of success have been reported. Light and electron microscopy were employed to better characterise the fibroadhesive scarring process taking place after implantation of a longitudinally microstructured type-I collagen scaffold into unilateral mid-cervical resection injuries of the adult rat spinal cord. At long survival times (10 weeks post-surgery), sheets of tightly packed cells (of uniform morphology) could be seen lining the inner surface of the repaired dura mater of lesion-only control animals, as well as forming a barrier along the implant-host interface of the scaffold-implanted animals. The highly uniform ultrastructural features of these scarring cells and their anatomical continuity with the local, reactive spinal nerve roots strongly suggest their identity to be perineurial-like cells. This novel aspect of the cellular composition of reactive spinal cord tissue highlights the increasingly complex nature of fibroadhesive scarring involved in traumatic injury, and particularly in response to the implantation of bioengineered collagen scaffolds.
Keywords: CNS-scarring; fibrotic encapsulation; implant interface; microstructured collagen scaffold; perineurial-like cells; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Dense fibroadhesive scarring and poor blood vessel-maturation hamper the integration of implanted collagen scaffolds in an experimental model of spinal cord injury.Biomed Mater. 2020 Feb 13;15(1):015012. doi: 10.1088/1748-605X/ab5e52. Biomed Mater. 2020. PMID: 31796648
-
Fibroadhesive scarring of grafted collagen scaffolds interferes with implant-host neural tissue integration and bridging in experimental spinal cord injury.Regen Biomater. 2019 Mar;6(2):75-87. doi: 10.1093/rb/rbz006. Epub 2019 Feb 4. Regen Biomater. 2019. PMID: 30967962 Free PMC article.
-
Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord.Brain Res. 2014 Oct 17;1585:37-50. doi: 10.1016/j.brainres.2014.08.041. Epub 2014 Sep 3. Brain Res. 2014. PMID: 25193604
-
Using extracellular matrix for regenerative medicine in the spinal cord.Biomaterials. 2013 Jul;34(21):4945-55. doi: 10.1016/j.biomaterials.2013.03.057. Epub 2013 Apr 15. Biomaterials. 2013. PMID: 23597407 Review.
-
Polymer scaffolds facilitate spinal cord injury repair.Acta Biomater. 2019 Apr 1;88:57-77. doi: 10.1016/j.actbio.2019.01.056. Epub 2019 Jan 31. Acta Biomater. 2019. PMID: 30710714 Review.
Cited by
-
In Vivo Assessment on Freeze-Cast Calcium Phosphate-Based Scaffolds with a Selective Cell/Tissue Ingrowth.ACS Appl Mater Interfaces. 2024 Oct 30;16(43):58326-58336. doi: 10.1021/acsami.4c12715. Epub 2024 Oct 21. ACS Appl Mater Interfaces. 2024. PMID: 39431911 Free PMC article.
-
The intriguing perineurial cells - an updated overview of their origin, structure, functions and implication in pathology.Rom J Morphol Embryol. 2024 Oct-Dec;65(4):567-574. doi: 10.47162/RJME.65.4.02. Rom J Morphol Embryol. 2024. PMID: 39957017 Free PMC article. Review.
References
-
- Bunge R.P., Puckett W.R., Becerra J.L., Marcillo A., Quencer R.M. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 1993;59:75–89. - PubMed
-
- Kakulas B., Taylor J. Pathology of injuries of the vertebral column and spinal cord. Handb. Clin. Neurol. 1992;17:21–51.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

