Phosphatidic Acid in Plant Hormonal Signaling: From Target Proteins to Membrane Conformations
- PMID: 35328648
- PMCID: PMC8954910
- DOI: 10.3390/ijms23063227
Phosphatidic Acid in Plant Hormonal Signaling: From Target Proteins to Membrane Conformations
Abstract
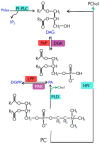
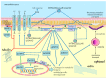
Cells sense a variety of extracellular signals balancing their metabolism and physiology according to changing growth conditions. Plasma membranes are the outermost informational barriers that render cells sensitive to regulatory inputs. Membranes are composed of different types of lipids that play not only structural but also informational roles. Hormones and other regulators are sensed by specific receptors leading to the activation of lipid metabolizing enzymes. These enzymes generate lipid second messengers. Among them, phosphatidic acid (PA) is a well-known intracellular messenger that regulates various cellular processes. This lipid affects the functional properties of cell membranes and binds to specific target proteins leading to either genomic (affecting transcriptome) or non-genomic responses. The subsequent biochemical, cellular and physiological reactions regulate plant growth, development and stress tolerance. In the present review, we focus on primary (genome-independent) signaling events triggered by rapid PA accumulation in plant cells and describe the functional role of PA in mediating response to hormones and hormone-like regulators. The contributions of individual lipid signaling enzymes to the formation of PA by specific stimuli are also discussed. We provide an overview of the current state of knowledge and future perspectives needed to decipher the mode of action of PA in the regulation of cell functions.
Keywords: autophagy; biologically active substance; diacylglycerol kinase; phosphatidic acid; phospholipase; phospholipid; signal transduction; targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Molecular, cellular, and physiological responses to phosphatidic acid formation in plants.J Exp Bot. 2011 Apr;62(7):2349-61. doi: 10.1093/jxb/err079. Epub 2011 Mar 23. J Exp Bot. 2011. PMID: 21430291 Review.
-
The phosphatidic acid paradox: Too many actions for one molecule class? Lessons from plants.Prog Lipid Res. 2018 Jul;71:43-53. doi: 10.1016/j.plipres.2018.05.003. Epub 2018 May 26. Prog Lipid Res. 2018. PMID: 29842906 Review.
-
Phospholipase D and phosphatidic acid in plant defence response: from protein-protein and lipid-protein interactions to hormone signalling.J Exp Bot. 2015 Apr;66(7):1721-36. doi: 10.1093/jxb/eru540. Epub 2015 Feb 13. J Exp Bot. 2015. PMID: 25680793 Free PMC article. Review.
-
Phospholipase D- and phosphatidic acid-mediated signaling in plants.Biochim Biophys Acta. 2009 Sep;1791(9):927-35. doi: 10.1016/j.bbalip.2009.02.017. Epub 2009 Mar 13. Biochim Biophys Acta. 2009. PMID: 19289179 Review.
-
[The role of phospholipase D in cellular signaling].Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2005 Oct;31(5):451-60. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2005. PMID: 16222086 Review. Chinese.
Cited by
-
High-resolution genome-wide association study of a large Czech collection of sweet cherry (Prunus avium L.) on fruit maturity and quality traits.Hortic Res. 2022 Oct 19;10(1):uhac233. doi: 10.1093/hr/uhac233. eCollection 2023. Hortic Res. 2022. PMID: 36643756 Free PMC article.
-
Plant PI-PLC signaling in stress and development.Plant Physiol. 2025 Feb 7;197(2):kiae534. doi: 10.1093/plphys/kiae534. Plant Physiol. 2025. PMID: 39928581 Free PMC article. Review.
-
Integrated Lipidomic and Transcriptomic Analysis Reveals Phospholipid Changes in Somatic Embryos of Picea asperata in Response to Partial Desiccation.Int J Mol Sci. 2022 Jun 10;23(12):6494. doi: 10.3390/ijms23126494. Int J Mol Sci. 2022. PMID: 35742942 Free PMC article.
-
Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity.bioRxiv [Preprint]. 2024 Mar 2:2024.03.01.582964. doi: 10.1101/2024.03.01.582964. bioRxiv. 2024. Update in: Nat Commun. 2024 Aug 15;15(1):7020. doi: 10.1038/s41467-024-51181-4. PMID: 38464030 Free PMC article. Updated. Preprint.
-
Phosphatidic acid signaling and function in nuclei.Prog Lipid Res. 2024 Jan;93:101267. doi: 10.1016/j.plipres.2023.101267. Epub 2023 Dec 26. Prog Lipid Res. 2024. PMID: 38154743 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

