Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat
- PMID: 35328650
- PMCID: PMC8952423
- DOI: 10.3390/ijms23063229
Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat
Abstract
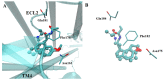
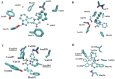
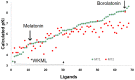
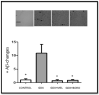
Preclinical and clinical evidence supports melatonin and its analogues as potential treatment for diseases involving cognitive deficit such as Alzheimer's disease. In this work, we evaluated by in silico studies a set of boron-containing melatonin analogues on MT1 and MT2 receptors. Then, we synthesized a compound (borolatonin) identified as potent agonist. After chemical characterization, its evaluation in a rat model with cognitive deficit showed that it induced ameliorative effects such as those induced by equimolar administration of melatonin in behavioral tests and in neuronal immunohistochemistry assays. Our results suggest the observed effects are by means of action on the melatonin system. Further studies are required to clarify the mechanism(s) of action, as the beneficial effects on disturbed memory by gonadectomy in male rats are attractive.
Keywords: Alzheimer; amyloid; androgen depletion; boron; boron-containing compounds; boroxazolidone; cognitive deficit; male rat; melatonin; melatonin receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Borolatonin limits cognitive deficit and neuron loss while increasing proBDNF in ovariectomised rats.Fundam Clin Pharmacol. 2024 Aug;38(4):730-741. doi: 10.1111/fcp.12997. Epub 2024 Feb 29. Fundam Clin Pharmacol. 2024. PMID: 38423984
-
Melatonin MT1 and MT2 Receptors Exhibit Distinct Effects in the Modulation of Body Temperature across the Light/Dark Cycle.Int J Mol Sci. 2019 May 17;20(10):2452. doi: 10.3390/ijms20102452. Int J Mol Sci. 2019. PMID: 31108968 Free PMC article.
-
Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: therapeutic implications for Alzheimer's disease.Neurosci Lett. 2014 Jul 25;576:84-7. doi: 10.1016/j.neulet.2014.05.056. Epub 2014 Jun 5. Neurosci Lett. 2014. PMID: 24909617
-
Docking studies for melatonin receptors.Expert Opin Drug Discov. 2018 Mar;13(3):241-248. doi: 10.1080/17460441.2018.1419184. Epub 2017 Dec 22. Expert Opin Drug Discov. 2018. PMID: 29271261 Review.
-
[Sites and mechanisms of action of melatonin in mammals: the MT1 and MT2 receptors].J Soc Biol. 2007;201(1):85-96. doi: 10.1051/jbio:2007010. J Soc Biol. 2007. PMID: 17762828 Review. French.
Cited by
-
Beneficial Effect of Melatonin Alone or in Combination with Glatiramer Acetate and Interferon β-1b on Experimental Autoimmune Encephalomyelitis.Molecules. 2022 Jun 30;27(13):4217. doi: 10.3390/molecules27134217. Molecules. 2022. PMID: 35807462 Free PMC article.
-
In silico identification of a biarylamine acting as agonist at human β3 adrenoceptors and exerting BRL37344-like effects on mouse metabolism.Naunyn Schmiedebergs Arch Pharmacol. 2024 Apr;397(4):2159-2170. doi: 10.1007/s00210-023-02753-6. Epub 2023 Oct 4. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37792048
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

