A Modular Composite Device of Poly(Ethylene Oxide)/Poly(Butylene Terephthalate) (PEOT/PBT) Nanofibers and Gelatin as a Dual Drug Delivery System for Local Therapy of Soft Tissue Tumors
- PMID: 35328661
- PMCID: PMC8948985
- DOI: 10.3390/ijms23063239
A Modular Composite Device of Poly(Ethylene Oxide)/Poly(Butylene Terephthalate) (PEOT/PBT) Nanofibers and Gelatin as a Dual Drug Delivery System for Local Therapy of Soft Tissue Tumors
Abstract
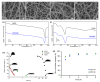
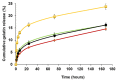
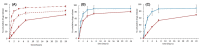
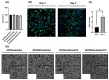
In the clinical management of solid tumors, the possibility to successfully couple the regeneration of injured tissues with the elimination of residual tumor cells left after surgery could open doors to new therapeutic strategies. In this work, we present a composite hydrogel-electrospun nanofiber scaffold, showing a modular architecture for the delivery of two pharmaceutics with distinct release profiles, that is potentially suitable for local therapy and post-surgical treatment of solid soft tumors. The composite was obtained by coupling gelatin hydrogels to poly(ethylene oxide)/poly(butylene terephthalate) block copolymer nanofibers. Results of the scaffolds' characterization, together with the analysis of gelatin and drug release kinetics, displayed the possibility to modulate the device architecture to control the release kinetics of the drugs, also providing evidence of their activity. In vitro analyses were also performed using a human epithelioid sarcoma cell line. Furthermore, publicly available expression datasets were interrogated. Confocal imaging showcased the nontoxicity of these devices in vitro. ELISA assays confirmed a modulation of IL-10 inflammation-related cytokine supporting the role of this device in tissue repair. In silico analysis confirmed the role of IL-10 in solid tumors including 262 patients affected by sarcoma as a negative prognostic marker for overall survival. In conclusion, the developed modular composite device may provide a key-enabling technology for the treatment of soft tissue sarcoma.
Keywords: PEOT/PBT; chemotherapy; composite scaffold; dual-drug delivery systems; electrospinning; gelatin; hydrogel; regenerative medicine; sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models.Biomatter. 2014;4:e955386. doi: 10.4161/21592527.2014.955386. Biomatter. 2014. PMID: 25482337 Free PMC article.
-
Biological and Tribological Assessment of Poly(Ethylene Oxide Terephthalate)/Poly(Butylene Terephthalate), Polycaprolactone, and Poly (L\DL) Lactic Acid Plotted Scaffolds for Skeletal Tissue Regeneration.Adv Healthc Mater. 2016 Jan 21;5(2):232-43. doi: 10.1002/adhm.201500067. Epub 2015 Nov 25. Adv Healthc Mater. 2016. PMID: 26775915
-
Melt Electrowriting of Elastic Scaffolds Using PEOT-PBT Multi-block Copolymer.Adv Healthc Mater. 2025 Jan;14(3):e2402914. doi: 10.1002/adhm.202402914. Epub 2024 Dec 10. Adv Healthc Mater. 2025. PMID: 39659166 Free PMC article.
-
Poly(ethylene terephthalate), Poly(butylene terephthalate), and Polystyrene Oligomers: Occurrence and Analysis in Food Contact Materials and Food.J Agric Food Chem. 2023 Feb 8;71(5):2244-2258. doi: 10.1021/acs.jafc.2c08558. Epub 2023 Jan 30. J Agric Food Chem. 2023. PMID: 36716125 Review.
-
Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment.Small. 2018 Jun 27:e1801183. doi: 10.1002/smll.201801183. Online ahead of print. Small. 2018. PMID: 29952070 Free PMC article. Review.
Cited by
-
3D Culture Modeling of Metastatic Breast Cancer Cells in Additive Manufactured Scaffolds.ACS Appl Mater Interfaces. 2022 Jun 22;14(24):28389-28402. doi: 10.1021/acsami.2c07492. Epub 2022 Jun 10. ACS Appl Mater Interfaces. 2022. PMID: 35687666 Free PMC article.
-
Gelatin-based anticancer drug delivery nanosystems: A mini review.Front Bioeng Biotechnol. 2023 Mar 21;11:1158749. doi: 10.3389/fbioe.2023.1158749. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37025360 Free PMC article. Review.
-
The emerging role of cancer nanotechnology in the panorama of sarcoma.Front Bioeng Biotechnol. 2022 Oct 17;10:953555. doi: 10.3389/fbioe.2022.953555. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36324885 Free PMC article. Review.
-
Advances in the Application of Electrospun Drug-Loaded Nanofibers in the Treatment of Oral Ulcers.Biomolecules. 2022 Sep 7;12(9):1254. doi: 10.3390/biom12091254. Biomolecules. 2022. PMID: 36139093 Free PMC article. Review.
-
Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome.Pharmaceutics. 2024 Aug 14;16(8):1066. doi: 10.3390/pharmaceutics16081066. Pharmaceutics. 2024. PMID: 39204410 Free PMC article.
References
-
- Gualandi C., Bloise N., Mauro N., Ferruti P., Manfredi A., Sampaolesi M., Liguori A., Laurita R., Gherardi M., Colombo V., et al. Poly-l-Lactic Acid Nanofi ber–Polyamidoamine Hydrogel Composites: Preparation, Properties, and Preliminary Evaluation as Scaffolds for Human Pluripotent Stem Cell Culturing. Macromol. Biosci. 2016;16:1533–1544. doi: 10.1002/mabi.201600061. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

