In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging
- PMID: 35328678
- PMCID: PMC8955704
- DOI: 10.3390/ijms23063258
In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging
Abstract
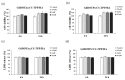
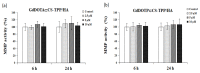
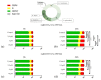
The aim of this study was to investigate the biocompatibility of contrast agents, such as gadolinium 1, 4, 7, 10 tetraazacyclo-dodecane tetraacetic acid (GdDOTA) and gadolinium dioctyl terephthalate (GdDOTP), encapsulated in a polymeric matrix containing chitosan and hyaluronic acid using RAW264.7 murine macrophages and human blood samples. The cell viability and cytotoxicity were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase (LDH) assays, while cell cycle analysis was determined in RAW264.7 cells using flow cytometry. The mitochondrial membrane potential (MMP), hemolytic index, complement activation, and thrombogenic potential of gadolinium (Gd) containing nanohydrogels were measured by fluorometric and spectrophotometric methods. Taken together, our results demonstrate the good bio- and hemocompatibility of chitosan-based nanohydrogels with the RAW264.7 cell line and human blood cells, suggesting that these could be used as injectable formulations for the magnetic resonance imaging diagnostic of lymph nodes.
Keywords: RAW 264.7 cell line; biocompatibility; chitosan; contrast agents; hemocompatibility; nanohydrogel.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

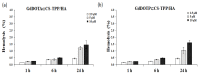
Similar articles
-
In Vivo Evaluation of Innovative Gadolinium-Based Contrast Agents Designed for Bioimaging Applications.Polymers (Basel). 2024 Apr 11;16(8):1064. doi: 10.3390/polym16081064. Polymers (Basel). 2024. PMID: 38674983 Free PMC article.
-
Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging.Nanomaterials (Basel). 2018 Mar 28;8(4):201. doi: 10.3390/nano8040201. Nanomaterials (Basel). 2018. PMID: 29597306 Free PMC article.
-
Unlocking the potential of biocompatible chitosan-hyaluronic acid nanogels labeled with fluorochromes: A promising step toward enhanced FRET bioimaging.Int J Biol Macromol. 2024 Dec;282(Pt 4):137063. doi: 10.1016/j.ijbiomac.2024.137063. Epub 2024 Oct 29. Int J Biol Macromol. 2024. PMID: 39481720
-
Integration of gadolinium in nanostructure for contrast enhanced-magnetic resonance imaging.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Jan;12(1):e1580. doi: 10.1002/wnan.1580. Epub 2019 Sep 5. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 31486295 Review.
-
Gadolinium(III)-based blood-pool contrast agents for magnetic resonance imaging: status and clinical potential.Expert Opin Drug Deliv. 2007 Mar;4(2):149-64. doi: 10.1517/17425247.4.2.149. Expert Opin Drug Deliv. 2007. PMID: 17335412 Review.
Cited by
-
In Vivo Evaluation of Innovative Gadolinium-Based Contrast Agents Designed for Bioimaging Applications.Polymers (Basel). 2024 Apr 11;16(8):1064. doi: 10.3390/polym16081064. Polymers (Basel). 2024. PMID: 38674983 Free PMC article.
-
Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections.Int J Mol Sci. 2024 Feb 17;25(4):2389. doi: 10.3390/ijms25042389. Int J Mol Sci. 2024. PMID: 38397066 Free PMC article.
-
Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives.Gels. 2024 Jul 18;10(7):477. doi: 10.3390/gels10070477. Gels. 2024. PMID: 39057500 Free PMC article.
-
Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action.Pharmaceuticals (Basel). 2023 Aug 4;16(8):1102. doi: 10.3390/ph16081102. Pharmaceuticals (Basel). 2023. PMID: 37631017 Free PMC article.
-
Triphenylphosphine Derivatives of Allylbenzenes Express Antitumor and Adjuvant Activity When Solubilized with Cyclodextrin-Based Formulations.Pharmaceuticals (Basel). 2023 Nov 26;16(12):1651. doi: 10.3390/ph16121651. Pharmaceuticals (Basel). 2023. PMID: 38139778 Free PMC article.
References
-
- Yanzhang W., Guanghua L., Zhihao Z., Zhixiong W., Zhao W. The risk of lymph node metastasis in gastric cancer conforming to indications of endoscopic resection and pylorus-preserving gastrectomy: A single-center retrospective study. BMC Cancer. 2021;21:1280. doi: 10.1186/s12885-021-09008-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

