Extracellular Vesicles as Signal Carriers in Malignant Thyroid Tumors?
- PMID: 35328683
- PMCID: PMC8955189
- DOI: 10.3390/ijms23063262
Extracellular Vesicles as Signal Carriers in Malignant Thyroid Tumors?
Abstract
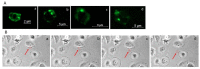
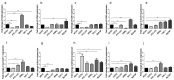
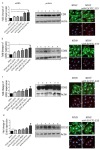
Extracellular vesicles (EVs) are small, membranous structures involved in intercellular communication. Here, we analyzed the effects of thyroid cancer-derived EVs on the properties of normal thyroid cells and cells contributing to the tumor microenvironment. EVs isolated from thyroid cancer cell lines (CGTH, FTC-133, 8505c, TPC-1 and BcPAP) were used for treatment of normal thyroid cells (NTHY), as well as monocytes and endothelial cells (HUVEC). EVs' size/number were analyzed by flow cytometry and confocal microscopy. Gene expression, protein level and localization were investigated by qRT-PCR, WB and ICC/IF, respectively. Proliferation, migration and tube formation were analyzed. When compared with NTHY, CGTH and BcPAP secreted significantly more EVs. Treatment of NTHY with cancer-derived EVs changed the expression of tetraspanin genes, but did not affect proliferation and migration. Cancer-derived EVs suppressed tube formation by endothelial cells and did not affect the phagocytic index of monocytes. The number of 6 μm size fraction of cancer-derived EVs correlated negatively with the CD63 and CD81 expression in NTHY cells, as well as positively with angiogenesis in vitro. Thyroid cancer-derived EVs can affect the expression of tetraspanins in normal thyroid cells. It is possible that 6 μm EVs contribute to the regulation of NTHY gene expression and angiogenesis.
Keywords: angiogenesis; extracellular vesicles; tetraspanins; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin.J Virol. 2019 Jan 4;93(2):e01850-18. doi: 10.1128/JVI.01850-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355691 Free PMC article.
-
Brain endothelial cells-derived extracellular vesicles overexpressing ECRG4 inhibit glioma proliferation through suppressing inflammation and angiogenesis.J Tissue Eng Regen Med. 2021 Dec;15(12):1162-1171. doi: 10.1002/term.3244. Epub 2021 Sep 29. J Tissue Eng Regen Med. 2021. PMID: 34551201
-
Exosomes increased angiogenesis in papillary thyroid cancer microenvironment.Endocr Relat Cancer. 2019 May;26(5):525-538. doi: 10.1530/ERC-19-0008. Endocr Relat Cancer. 2019. PMID: 30870812
-
The role of extracellular vesicles from different origin in the microenvironment of head and neck cancers.Mol Cancer. 2019 Apr 6;18(1):83. doi: 10.1186/s12943-019-0985-3. Mol Cancer. 2019. PMID: 30954079 Free PMC article. Review.
-
Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling.Cell Commun Signal. 2022 Jun 25;20(1):97. doi: 10.1186/s12964-022-00904-5. Cell Commun Signal. 2022. PMID: 35752798 Free PMC article. Review.
Cited by
-
Role of Extracellular Vesicles in Thyroid Physiology and Diseases: Implications for Diagnosis and Treatment.Biomedicines. 2022 Oct 15;10(10):2585. doi: 10.3390/biomedicines10102585. Biomedicines. 2022. PMID: 36289847 Free PMC article. Review.
-
Agrin is a novel oncogenic protein in thyroid cancer.Oncol Lett. 2023 Sep 25;26(5):483. doi: 10.3892/ol.2023.14070. eCollection 2023 Nov. Oncol Lett. 2023. PMID: 37818129 Free PMC article.
References
-
- Gervasoni J.E., Cady B. Endocrine Tumors. In: Bertino J.R., editor. Encyclopedia of Cancer. 2nd ed. Academic Press; New York, NY, USA: 2002. pp. 135–143.
-
- Greco A., Miranda C., Borrello M.G., Pierotti M.A. Chapter 16-Thyroid Cancer. In: Dellaire G., Berman J.N., Arceci R.J., editors. Cancer Genomics. Academic Press; Boston, MA, USA: 2014. pp. 265–280.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

