Polymeric Hydrogels for In Vitro 3D Ovarian Cancer Modeling
- PMID: 35328686
- PMCID: PMC8954571
- DOI: 10.3390/ijms23063265
Polymeric Hydrogels for In Vitro 3D Ovarian Cancer Modeling
Abstract
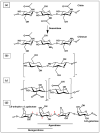
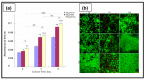
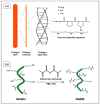
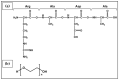
Ovarian cancer (OC) grows and interacts constantly with a complex microenvironment, in which immune cells, fibroblasts, blood vessels, signal molecules and the extracellular matrix (ECM) coexist. This heterogeneous environment provides structural and biochemical support to the surrounding cells and undergoes constant and dynamic remodeling that actively promotes tumor initiation, progression, and metastasis. Despite the fact that traditional 2D cell culture systems have led to relevant medical advances in cancer research, 3D cell culture models could open new possibilities for the development of an in vitro tumor microenvironment more closely reproducing that observed in vivo. The implementation of materials science and technology into cancer research has enabled significant progress in the study of cancer progression and drug screening, through the development of polymeric scaffold-based 3D models closely recapitulating the physiopathological features of native tumor tissue. This article provides an overview of state-of-the-art in vitro tumor models with a particular focus on 3D OC cell culture in pre-clinical studies. The most representative OC models described in the literature are presented with a focus on hydrogel-based scaffolds, which guarantee soft tissue-like physical properties as well as a suitable 3D microenvironment for cell growth. Hydrogel-forming polymers of either natural or synthetic origin investigated in this context are described by highlighting their source of extraction, physical-chemical properties, and application for 3D ovarian cancer cell culture.
Keywords: 3D cell culture; hydrogel; ovarian cancer; polymer; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Ovarian Cancer. [(accessed on 15 February 2022)]; Available online: https://seer.cancer.gov.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

