Differential Metabotypes in Synovial Fibroblasts and Synovial Fluid in Hip Osteoarthritis Patients Support Inflammatory Responses
- PMID: 35328687
- PMCID: PMC8950319
- DOI: 10.3390/ijms23063266
Differential Metabotypes in Synovial Fibroblasts and Synovial Fluid in Hip Osteoarthritis Patients Support Inflammatory Responses
Abstract
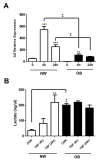
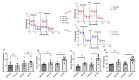
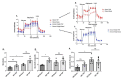
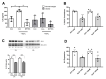
Changes in cellular metabolism have been implicated in mediating the activated fibroblast phenotype in a number of chronic inflammatory disorders, including pulmonary fibrosis, renal disease and rheumatoid arthritis. The aim of this study was therefore to characterise the metabolic profile of synovial joint fluid and synovial fibroblasts under both basal and inflammatory conditions in a cohort of obese and normal-weight hip OA patients. Furthermore, we sought to ascertain whether modulation of a metabolic pathway in OA synovial fibroblasts could alter their inflammatory activity. Synovium and synovial fluid was obtained from hip OA patients, who were either of normal-weight or obese and were undergoing elective joint replacement surgery. The synovial fluid metabolome was determined by 1H NMR spectroscopy. The metabolic profile of isolated synovial fibroblasts in vitro was characterised by lactate secretion, oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) using the Seahorse XF Analyser. The effects of a small molecule pharmacological inhibitor and siRNA targeted at glutaminase-1 (GLS1) were assessed to probe the role of glutamine metabolism in OA synovial fibroblast function. Obese OA patient synovial fluid (n = 5) exhibited a different metabotype, compared to normal-weight patient fluid (n = 6), with significantly increased levels of 1, 3-dimethylurate, N-Nitrosodimethylamine, succinate, tyrosine, pyruvate, glucose, glycine and lactate, and enrichment of the glutamine-glutamate metabolic pathway, which correlated with increasing adiposity. In vitro, isolated obese OA fibroblasts exhibited greater basal lactate secretion and aerobic glycolysis, and increased mitochondrial respiration when stimulated with pro-inflammatory cytokine TNFα, compared to fibroblasts from normal-weight patients. Inhibition of GLS1 attenuated the TNFα-induced expression and secretion of IL-6 in OA synovial fibroblasts. These findings suggest that altered cellular metabolism underpins the inflammatory phenotype of OA fibroblasts, and that targeted inhibition of glutamine-glutamate metabolism may provide a route to reducing the pathological effects of joint inflammation in OA patients who are obese.
Keywords: IL6; glutamine; inflammation; metabolism; osteoarthritis; synovial fibroblast.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the study.
Figures

Similar articles
-
Regulation of the Inflammatory Synovial Fibroblast Phenotype by Metastasis-Associated Lung Adenocarcinoma Transcript 1 Long Noncoding RNA in Obese Patients With Osteoarthritis.Arthritis Rheumatol. 2020 Apr;72(4):609-619. doi: 10.1002/art.41158. Epub 2020 Feb 27. Arthritis Rheumatol. 2020. PMID: 31682073
-
IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity.Sci Rep. 2017 Jun 14;7(1):3451. doi: 10.1038/s41598-017-03759-w. Sci Rep. 2017. PMID: 28615667 Free PMC article.
-
High levels of TDO2 in relation to pro-inflammatory cytokines in synovium and synovial fluid of patients with osteoarthritis.BMC Musculoskelet Disord. 2022 Jun 22;23(1):604. doi: 10.1186/s12891-022-05567-4. BMC Musculoskelet Disord. 2022. PMID: 35733134 Free PMC article.
-
Obesity defined molecular endotypes in the synovium of patients with osteoarthritis provides a rationale for therapeutic targeting of fibroblast subsets.Clin Transl Med. 2023 Apr;13(4):e1232. doi: 10.1002/ctm2.1232. Clin Transl Med. 2023. PMID: 37006170 Free PMC article.
-
Metabolic dysfunction and inflammatory disease: the role of stromal fibroblasts.FEBS J. 2021 Oct;288(19):5555-5568. doi: 10.1111/febs.15644. Epub 2020 Dec 17. FEBS J. 2021. PMID: 33251764 Free PMC article. Review.
Cited by
-
Synovial fluid as a complex molecular pool contributing to knee osteoarthritis.Nat Rev Rheumatol. 2025 Aug;21(8):447-464. doi: 10.1038/s41584-025-01271-4. Epub 2025 Jul 7. Nat Rev Rheumatol. 2025. PMID: 40624394 Review.
-
Association of glutamine metabolites in synovial fluid with the severity of temporomandibular joint osteoarthritis.BMC Musculoskelet Disord. 2025 Jul 5;26(1):653. doi: 10.1186/s12891-025-08898-0. BMC Musculoskelet Disord. 2025. PMID: 40618069 Free PMC article.
-
Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research.Int J Mol Sci. 2025 Jul 11;26(14):6665. doi: 10.3390/ijms26146665. Int J Mol Sci. 2025. PMID: 40724912 Free PMC article.
-
Increase in TPSB2 and TPSD1 Expression in Synovium of Hip Osteoarthritis Patients Who Are Overweight.Int J Mol Sci. 2023 Jul 16;24(14):11532. doi: 10.3390/ijms241411532. Int J Mol Sci. 2023. PMID: 37511292 Free PMC article.
-
Cross-species transcriptomics identifies obesity associated genes between human and mouse studies.J Transl Med. 2024 Jun 25;22(1):592. doi: 10.1186/s12967-024-05414-1. J Transl Med. 2024. PMID: 38918843 Free PMC article.
References
-
- Jones S.W., Brockbank S.M., Clements K.M., Le Good N., Campbell D., Read S.J., Needham M.R., Newham P. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr. Cartil. 2009;17:124–131. doi: 10.1016/j.joca.2008.05.001. - DOI - PubMed
-
- Jones S.W., Brockbank S.M., Mobbs M.L., Le Good N.J., Soma-Haddrick S., Heuze A.J., Langham C.J., Timms D., Newham P., Needham M.R. The orphan G-protein coupled receptor RDC1: Evidence for a role in chondrocyte hypertrophy and articular cartilage matrix turnover. Osteoarthr. Cartil. 2006;14:597–608. doi: 10.1016/j.joca.2006.01.007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

