Methylation of Subtelomeric Chromatin Modifies the Expression of the lncRNA TERRA, Disturbing Telomere Homeostasis
- PMID: 35328692
- PMCID: PMC8955364
- DOI: 10.3390/ijms23063271
Methylation of Subtelomeric Chromatin Modifies the Expression of the lncRNA TERRA, Disturbing Telomere Homeostasis
Abstract
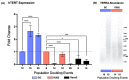
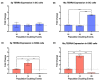
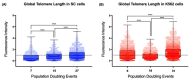
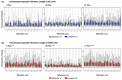
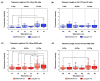
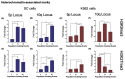
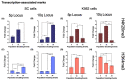
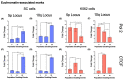
The long noncoding RNA (lncRNA) telomeric repeat-containing RNA (TERRA) has been associated with telomeric homeostasis, telomerase recruitment, and the process of chromosome healing; nevertheless, the impact of this association has not been investigated during the carcinogenic process. Determining whether changes in TERRA expression are a cause or a consequence of cell transformation is a complex task because studies are usually carried out using either cancerous cells or tumor samples. To determine the role of this lncRNA in cellular aging and chromosome healing, we evaluated telomeric integrity and TERRA expression during the establishment of a clone of untransformed myeloid cells. We found that reduced expression of TERRA disturbed the telomeric homeostasis of certain loci, but the expression of the lncRNA was affected only when the methylation of subtelomeric bivalent chromatin domains was compromised. We conclude that the disruption in TERRA homeostasis is a consequence of cellular transformation and that changes in its expression profile can lead to telomeric and genomic instability.
Keywords: TERRA; hTERT; lncRNA; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Telomeric Chromatin and TERRA.J Mol Biol. 2020 Jul 10;432(15):4244-4256. doi: 10.1016/j.jmb.2020.03.003. Epub 2020 Mar 7. J Mol Biol. 2020. PMID: 32151584 Review.
-
Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity.Front Genet. 2015 Apr 14;6:143. doi: 10.3389/fgene.2015.00143. eCollection 2015. Front Genet. 2015. PMID: 25926849 Free PMC article. Review.
-
DNA methylation mediated up-regulation of TERRA non-coding RNA is coincident with elongated telomeres in the human placenta.Mol Hum Reprod. 2016 Nov;22(11):791-799. doi: 10.1093/molehr/gaw053. Epub 2016 Sep 7. Mol Hum Reprod. 2016. PMID: 27604461
-
Telomeric noncoding RNA: telomeric repeat-containing RNA in telomere biology.Wiley Interdiscip Rev RNA. 2014 May-Jun;5(3):407-19. doi: 10.1002/wrna.1220. Epub 2014 Feb 12. Wiley Interdiscip Rev RNA. 2014. PMID: 24523222 Review.
-
Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis.BMC Pulm Med. 2017 Dec 2;17(1):163. doi: 10.1186/s12890-017-0516-1. BMC Pulm Med. 2017. PMID: 29197377 Free PMC article.
Cited by
-
LncRNAs-associated to genomic instability: A barrier to cancer therapy effectiveness.Front Genet. 2022 Nov 21;13:984329. doi: 10.3389/fgene.2022.984329. eCollection 2022. Front Genet. 2022. PMID: 36479250 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

