Molecular Mechanism Based on Histopathology, Antioxidant System and Transcriptomic Profiles in Heat Stress Response in the Gills of Japanese Flounder
- PMID: 35328705
- PMCID: PMC8955770
- DOI: 10.3390/ijms23063286
Molecular Mechanism Based on Histopathology, Antioxidant System and Transcriptomic Profiles in Heat Stress Response in the Gills of Japanese Flounder
Abstract
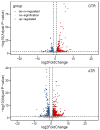
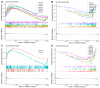
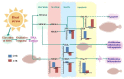
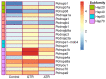
As an economically important flatfish in Asia, Japanese flounder is threatened by continuously rising temperatures due to global warming. To understand the molecular responses of this species to temperature stress, adult Japanese flounder individuals were treated with two kinds of heat stress-a gradual temperature rise (GTR) and an abrupt temperature rise (ATR)-in aquaria under experimental conditions. Changes in histopathology, programmed cell death levels and the oxidative stress status of gills were investigated. Histopathology showed that the damage caused by ATR stress was more serious. TUNEL signals confirmed this result, showing more programmed cell death in the ATR group. In addition, reactive oxygen species (ROS) levels and the 8-O-hDG contents of both the GTR and ATR groups increased significantly, and the total superoxide dismutase (T-SOD) activities and total antioxidant capacity (T-AOC) levels decreased in the two stressed groups, which showed damage to antioxidant systems. Meanwhile, RNA-seq was utilized to illustrate the molecular mechanisms underyling gill damage. Compared to the control group of 18 °C, 507 differentially expressed genes (DEGs) were screened in the GTR group; 341 were up-regulated and 166 were down-regulated, and pathway enrichment analysis indicated that they were involved in regulation and adaptation, including chaperone and folding catalyst pathways, the mitogen-activated protein kinase signaling (MAPK) pathway and DNA replication protein pathways. After ATR stress, 1070 DEGs were identified, 627 were up-regulated and 423 were down-regulated, and most DEGs were involved in chaperone and folding catalyst and DNA-related pathways, such as DNA replication proteins and nucleotide excision repair. The annotation of DEGs showed the great importance of heat shock proteins (HSPs) in protecting Japanese flounder from heat stress injury; 12 hsp genes were found after GTR, while 5 hsp genes were found after ATR. In summary, our study records gill dysfunction after heat stress, with different response patterns observed in the two experimental designs; chaperones were activated to defend heat stress after GTR, while replication was almost abandoned due to the severe damage consequent on ATR stress.
Keywords: Japanese flounder; gill; heat stress; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bagnyukova T., Lushchak O., Storey K.B., Lushchak V. Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 C. J. Therm. Biol. 2007;32:227–234. doi: 10.1016/j.jtherbio.2007.01.004. - DOI
-
- López-Olmeda J., Sánchez-Vázquez F. Thermal biology of zebrafish (Danio rerio) J. Therm. Biol. 2011;36:91–104. doi: 10.1016/j.jtherbio.2010.12.005. - DOI
-
- Sudo R., Tosaka R., Ijiri S., Adachi S., Suetake H., Suzuki Y., Horie N., Tanaka S., Aoyama J., Tsukamoto K. Effect of temperature decrease on oocyte development, sex steroids, and gonadotropin β-subunit mRNA expression levels in female Japanese eel Anguilla japonica. Fish. Sci. 2011;77:575–582. doi: 10.1007/s12562-011-0358-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

