Exogenous SA Affects Rice Seed Germination under Salt Stress by Regulating Na+/K+ Balance and Endogenous GAs and ABA Homeostasis
- PMID: 35328712
- PMCID: PMC8952856
- DOI: 10.3390/ijms23063293
Exogenous SA Affects Rice Seed Germination under Salt Stress by Regulating Na+/K+ Balance and Endogenous GAs and ABA Homeostasis
Abstract
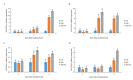
Salinity reduces agricultural productivity majorly by inhibiting seed germination. Exogenous salicylic acid (SA) can prevent the harm caused to rice by salinity, but the mechanisms by which it promotes rice seed germination under salt stress are unclear. In this study, the inhibition of germination in salt-sensitive Nipponbare under salt stress was greater than that in salt-tolerant Huaidao 5. Treatment with exogenous SA significantly improved germination of Nipponbare, but had little effect on Huaidao 5. The effects of exogenous SA on ion balance, metabolism of reactive oxygen species (ROS), hormone homeostasis, starch hydrolysis, and other physiological processes involved in seed germination of rice under salt stress were investigated. Under salt stress, Na+ content and the Na+/K+ ratio in rice seeds increased sharply. Seeds were subjected to ion pressure, which led to massive accumulation of H2O2, O2-, and malonaldehyde (MDA); imbalanced endogenous hormone homeostasis; decreased gibberellic acid (GA1 and GA4) content; increased abscisic acid (ABA) content; inhibition of α-amylase (EC 3.2.1.1) activity; and slowed starch hydrolysis rate, all which eventually led to the inhibition of the germination of rice seeds. Exogenous SA could effectively enhance the expression of OsHKT1;1, OsHKT1;5, OsHKT2;1 and OsSOS1 to reduce the absorption of Na+ by seeds; reduce the Na+/K+ ratio; improve the activities of SOD, POD, and CAT; reduce the accumulation of H2O2, O2-, and MDA; enhance the expression of the GA biosynthetic genes OsGA20ox1 and OsGA3ox2; inhibit the expression of the ABA biosynthetic gene OsNCED5; increase GA1 and GA4 content; reduce ABA content; improve α-amylase activity, and increase the content of soluble sugars. In summary, exogenous SA can alleviate ion toxicity by reducing Na+ content, thereby helping to maintain ROS and hormone homeostasis, promote starch hydrolysis, and provide sufficient energy for seed germination, all of which ultimately improves rice seed germination under salt stress. This study presents a feasible means for improving the germination of direct-seeded rice in saline soil.
Keywords: germination; hormone homeostasis; ion balance; rice; salicylic acid; salt stress.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



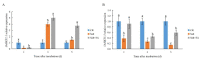





Similar articles
-
Sulforaphane modulates Na+/K+ homeostasis and hormonal balance in rice under salt stress by regulating OsHKT1 and OsHKT5 expression.Int J Biol Macromol. 2025 May;309(Pt 1):142783. doi: 10.1016/j.ijbiomac.2025.142783. Epub 2025 Apr 2. Int J Biol Macromol. 2025. PMID: 40185445
-
Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress.Plant Signal Behav. 2019;14(10):e1644595. doi: 10.1080/15592324.2019.1644595. Epub 2019 Jul 22. Plant Signal Behav. 2019. PMID: 31331225 Free PMC article.
-
Drying temperature affects rice seed vigor via gibberellin, abscisic acid, and antioxidant enzyme metabolism.J Zhejiang Univ Sci B. 2020 Oct.;21(10):796-810. doi: 10.1631/jzus.B2000297. J Zhejiang Univ Sci B. 2020. PMID: 33043645 Free PMC article.
-
The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination.Int J Mol Sci. 2019 Jan 21;20(2):450. doi: 10.3390/ijms20020450. Int J Mol Sci. 2019. PMID: 30669630 Free PMC article. Review.
-
Melatonin as a key regulator in seed germination under abiotic stress.J Pineal Res. 2024 Jan;76(1):e12937. doi: 10.1111/jpi.12937. J Pineal Res. 2024. PMID: 38241678 Review.
Cited by
-
CRISPR/Cas genome editing improves abiotic and biotic stress tolerance of crops.Front Genome Ed. 2022 Sep 7;4:987817. doi: 10.3389/fgeed.2022.987817. eCollection 2022. Front Genome Ed. 2022. PMID: 36188128 Free PMC article. Review.
-
Unfolding molecular switches for salt stress resilience in soybean: recent advances and prospects for salt-tolerant smart plant production.Front Plant Sci. 2023 Apr 19;14:1162014. doi: 10.3389/fpls.2023.1162014. eCollection 2023. Front Plant Sci. 2023. PMID: 37152141 Free PMC article. Review.
-
Investigating how reproductive traits in rice respond to abiotic stress.J Exp Bot. 2025 May 27;76(8):2064-2080. doi: 10.1093/jxb/eraf031. J Exp Bot. 2025. PMID: 39876691 Free PMC article. Review.
-
CRISPR/Cas genome editing system and its application in potato.Front Genet. 2023 Feb 13;14:1017388. doi: 10.3389/fgene.2023.1017388. eCollection 2023. Front Genet. 2023. PMID: 36861125 Free PMC article. Review.
-
Caffeic Acid O-Methyltransferase Gene Family in Mango (Mangifera indica L.) with Transcriptional Analysis under Biotic and Abiotic Stresses and the Role of MiCOMT1 in Salt Tolerance.Int J Mol Sci. 2024 Feb 24;25(5):2639. doi: 10.3390/ijms25052639. Int J Mol Sci. 2024. PMID: 38473886 Free PMC article.
References
-
- Rasheed F., Anjum N.A., Masood A., Sofo A., Khan N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020:1–14. doi: 10.1007/s00344-020-10257-3. - DOI
-
- Qadir M., Quillérou E., Nangia V., Murtaza G., Singh M., Thomas R.J., Drechsel P., Noble A.D. Economics of salt-induced land degradation and restoration. Nat. Res. Forum. 2014;38:282–295. doi: 10.1111/1477-8947.12054. - DOI
-
- Jamil A., Riaz S., Ashraf M., Foolad M.R. Gene expression profling of plants under salt stress. Crit. Rev. Plant Sci. 2011;30:435–458. doi: 10.1080/07352689.2011.605739. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

