Interactions between the Re-Emerging Pathogen Corynebacterium diphtheriae and Host Cells
- PMID: 35328715
- PMCID: PMC8952647
- DOI: 10.3390/ijms23063298
Interactions between the Re-Emerging Pathogen Corynebacterium diphtheriae and Host Cells
Abstract
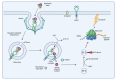
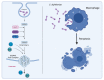
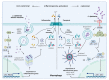
Corynebacterium diphtheriae, the etiological agent of diphtheria, is a re-emerging pathogen, responsible for several thousand deaths per year. In addition to diphtheria, systemic infections, often by non-toxigenic strains, are increasingly observed. This indicates that besides the well-studied and highly potent diphtheria toxin, various other virulence factors may influence the progression of the infection. This review focuses on the known components of C. diphtheriae responsible for adhesion, invasion, inflammation, and cell death, as well as on the cellular signaling pathways activated upon infection.
Keywords: Shiga-like toxin; apoptosis; diphtheria; diphtheria toxin; mycolic acids; necrosis; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
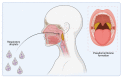
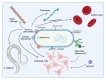
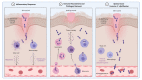
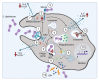

References
-
- Löffler F. Untersuchungen über die Bedeutung der Mikroorganismen für die Entstehung der Diphtherie beim Menschen, bei der Taube und beim Kalbe. Mitt. Dem Kais. Gesundh. 1884;2:421–499.
-
- Burkovski A. Diphtheria and its etiological agents. In: Burkovski A., editor. Corynebacterium diphtheriae and Related Toxigenic Species. Springer; Dordrecht, The Netherlands: 2014. pp. 1–14.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

