p62 Promotes the Mitochondrial Localization of p53 through Its UBA Domain and Participates in Regulating the Sensitivity of Ovarian Cancer Cells to Cisplatin
- PMID: 35328718
- PMCID: PMC8949157
- DOI: 10.3390/ijms23063290
p62 Promotes the Mitochondrial Localization of p53 through Its UBA Domain and Participates in Regulating the Sensitivity of Ovarian Cancer Cells to Cisplatin
Abstract
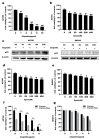
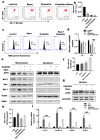
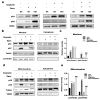
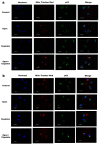
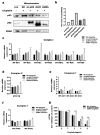
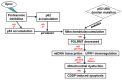
Chemotherapeutic drug-induced p53-dependent crosstalk among tumor cells affects the sensitivity of tumor cells to chemotherapeutic drugs, contributing to chemoresistance. Therefore, pharmacological targeting of p53 may contribute to overcoming drug resistance. The localization of p53 is closely related to its function. Thus, we assessed the effect of p62 on the coordination of p53 mitochondrial localization under chemotherapeutic drug treatment in ovarian cancer cells. We found that the combined use of the proteasome inhibitor epoxomicin and cisplatin led to the accumulation of p53 and sequestosome1(p62) in the mitochondria, downregulated mitochondrial DNA (mtDNA) transcription, inhibited mitochondrial functions, and ultimately promoted apoptosis by enhancing cisplatin sensitivity in ovarian cancer cells. Moreover, the ubiquitin-associated (UBA) domain of p62 was involved in regulating the mitochondrial localization of p53. Our findings suggest that the interaction between p62 and p53 may be a mechanism that determines the fate of tumor cells. In conclusion, p62 coordinated the mitochondrial localization of p53 through its UBA domain, inhibited mtDNA transcription, downregulated mitochondrial function, and promoted ovarian cancer cell death. Our study demonstrates the important role of p53 localization in tumor cell survival and apoptosis, and provides new insights into understanding the anti-tumor mechanism of targeting the ubiquitin-proteasome system in tumor cells.
Keywords: drug resistance; mitochondria; ovarian cancer; p53; p62/SQSTM1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The cisplatin-induced lncRNA PANDAR dictates the chemoresistance of ovarian cancer via regulating SFRS2-mediated p53 phosphorylation.Cell Death Dis. 2018 Oct 30;9(11):1103. doi: 10.1038/s41419-018-1148-y. Cell Death Dis. 2018. PMID: 30375398 Free PMC article.
-
Prohibitin 1 interacts with p53 in the regulation of mitochondrial dynamics and chemoresistance in gynecologic cancers.J Ovarian Res. 2022 Jun 7;15(1):70. doi: 10.1186/s13048-022-00999-x. J Ovarian Res. 2022. PMID: 35668443 Free PMC article.
-
An Experimentally Induced Mutation in the UBA Domain of p62 Changes the Sensitivity of Cisplatin by Up-Regulating HK2 Localisation on the Mitochondria and Increasing Mitophagy in A2780 Ovarian Cancer Cells.Int J Mol Sci. 2021 Apr 13;22(8):3983. doi: 10.3390/ijms22083983. Int J Mol Sci. 2021. PMID: 33924293 Free PMC article.
-
Novel factors of cisplatin resistance in epithelial ovarian tumours.Adv Med Sci. 2025 Mar;70(1):94-102. doi: 10.1016/j.advms.2025.01.005. Epub 2025 Jan 27. Adv Med Sci. 2025. PMID: 39880191 Review.
-
Molecular mechanisms of platinum‑based chemotherapy resistance in ovarian cancer (Review).Oncol Rep. 2022 Apr;47(4):82. doi: 10.3892/or.2022.8293. Epub 2022 Feb 25. Oncol Rep. 2022. PMID: 35211759 Free PMC article. Review.
Cited by
-
Nrf2 and Parkin-Hsc70 regulate the expression and protein stability of p62/SQSTM1 under hypoxia.Sci Rep. 2022 Dec 8;12(1):21265. doi: 10.1038/s41598-022-25784-0. Sci Rep. 2022. PMID: 36481701 Free PMC article.
-
Unlocking the mitochondrial functional code: unraveling the pathogenesis of ovarian cancer and innovative targets to inhibit malignant behavior.J Transl Med. 2025 Jul 24;23(1):819. doi: 10.1186/s12967-025-06840-5. J Transl Med. 2025. PMID: 40707948 Free PMC article. Review.
-
Role of autophagy and its regulation by noncoding RNAs in ovarian cancer.Exp Biol Med (Maywood). 2023 Jun;248(12):1001-1012. doi: 10.1177/15353702231151958. Epub 2023 Feb 18. Exp Biol Med (Maywood). 2023. PMID: 36803116 Free PMC article. Review.
-
Unraveling role of ubiquitination in drug resistance of gynecological cancer.Am J Cancer Res. 2024 May 15;14(5):2523-2537. doi: 10.62347/WYKZ9784. eCollection 2024. Am J Cancer Res. 2024. PMID: 38859858 Free PMC article. Review.
-
Mitochondrial DNA-targeted therapy: A novel approach to combat cancer.Cell Insight. 2023 Jul 22;2(4):100113. doi: 10.1016/j.cellin.2023.100113. eCollection 2023 Aug. Cell Insight. 2023. PMID: 37554301 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

