Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles
- PMID: 35328731
- PMCID: PMC8954933
- DOI: 10.3390/ijms23063311
Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles
Abstract
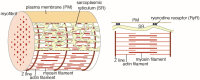
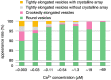
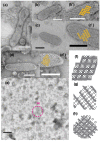
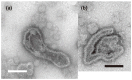
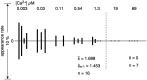
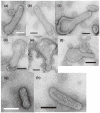
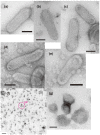
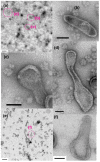
The Ca2+-ATPase is an integral transmembrane Ca2+ pump of the sarcoplasmic reticulum (SR). Crystallization of the cytoplasmic surface ATPase molecules of isolated scallop SR vesicles was studied at various calcium concentrations by negative stain electron microscopy. In the absence of ATP, round SR vesicles displaying an assembly of small crystalline patches of ATPase molecules were observed at 18 µM [Ca2+]. These partly transformed into tightly elongated vesicles containing ATPase crystalline arrays at low [Ca2+] (≤1.3 µM). The arrays were classified as ''tetramer'', "two-rail" (like a railroad) and ''monomer''. Their crystallinity was low, and they were unstable. In the presence of ATP (5 mM) at a low [Ca2+] of ~0.002 µM, "two-rail" arrays of high crystallinity appeared more frequently in the tightly elongated vesicles and the distinct tetramer arrays disappeared. During prolonged (~2.5 h) incubation, ATP was consumed and tetramer arrays reappeared. A specific ATPase inhibitor, thapsigargin, prevented both crystal formation and vesicle elongation in the presence of ATP. Together with the second part of this study, these data suggest that the ATPase forms tetramer units and longer tetramer crystalline arrays to elongate SR vesicles, and that the arrays transform into more stable "two-rail" forms in the presence of ATP at low [Ca2+].
Keywords: ATP; Ca2+-ATPase; cell dynamics; cell morphology; membrane endoskeleton; sarcoplasmic reticulum; scallop; thapsigargin; transmission microscopy; two-dimensional crystallization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ca2+ Dependent Formation/Collapse of Cylindrical Ca2+-ATPase Crystals in Scallop Sarcoplasmic Reticulum (SR) Vesicles: A Possible Dynamic Role of SR in Regulation of Muscle Contraction.Int J Mol Sci. 2023 Apr 11;24(8):7080. doi: 10.3390/ijms24087080. Int J Mol Sci. 2023. PMID: 37108240 Free PMC article.
-
Ca2+-ATPase Molecules as a Calcium-Sensitive Membrane-Endoskeleton of Sarcoplasmic Reticulum.Int J Mol Sci. 2021 Mar 5;22(5):2624. doi: 10.3390/ijms22052624. Int J Mol Sci. 2021. PMID: 33807779 Free PMC article.
-
Effect of Ca2+ on the dimeric structure of scallop sarcoplasmic reticulum.J Cell Biol. 1989 Feb;108(2):511-20. doi: 10.1083/jcb.108.2.511. J Cell Biol. 1989. PMID: 2521860 Free PMC article.
-
Ca(2+ )transport by the sarcoplasmic reticulum Ca(2+)-ATPase in sea cucumber (Ludwigothurea grisea) muscle.J Exp Biol. 2001 Mar;204(Pt 5):909-21. doi: 10.1242/jeb.204.5.909. J Exp Biol. 2001. PMID: 11171414 Review.
-
A comparative study of the Ca2+-Mg2+ dependent ATPase from skeletal muscles of young, adult and old rats.Mech Ageing Dev. 1989 Aug;49(2):105-117. doi: 10.1016/0047-6374(89)90094-8. Mech Ageing Dev. 1989. PMID: 2529400 Review.
Cited by
-
Ca2+ Dependent Formation/Collapse of Cylindrical Ca2+-ATPase Crystals in Scallop Sarcoplasmic Reticulum (SR) Vesicles: A Possible Dynamic Role of SR in Regulation of Muscle Contraction.Int J Mol Sci. 2023 Apr 11;24(8):7080. doi: 10.3390/ijms24087080. Int J Mol Sci. 2023. PMID: 37108240 Free PMC article.
References
-
- De Meis L., Inesi G. The transport of calcium by sarcoplasmic reticulum and various microsomal preparations. In: Carafoli E., editor. Membrane Transport of Calcium. Academic Press, Inc.; Orland, FL, USA: 1982. pp. 141–186.
-
- Hasselbach W. Relaxing factor and the relaxation of muscle. In: Bupler J.A.V., Huxley H.E., editors. Progress in Biophysics and Molecular Biology. Pergamon Press; London, UK: 1964. pp. 167–222.
-
- Ebashi S., Endo M. Calcium ion and muscle contraction. In: Bupler J.A.V., Huxley H.E., editors. Progress in Biophysics and Molecular Biology. Pergamon Press; London, UK: 1968. pp. 123–183. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

