Role of C-Terminal Domain and Membrane Potential in the Mobility of Kv1.3 Channels in Immune Synapse Forming T Cells
- PMID: 35328733
- PMCID: PMC8952507
- DOI: 10.3390/ijms23063313
Role of C-Terminal Domain and Membrane Potential in the Mobility of Kv1.3 Channels in Immune Synapse Forming T Cells
Abstract
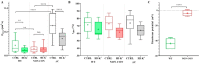
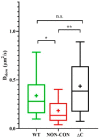
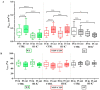
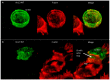
Voltage-gated Kv1.3 potassium channels are essential for maintaining negative membrane potential during T-cell activation. They interact with membrane-associated guanylate kinases (MAGUK-s) via their C-terminus and with TCR/CD3, leading to enrichment at the immunological synapse (IS). Molecular interactions and mobility may impact each other and the function of these proteins. We aimed to identify molecular determinants of Kv1.3 mobility, applying fluorescence correlation spectroscopy on human Jurkat T-cells expressing WT, C-terminally truncated (ΔC), and non-conducting mutants of mGFP-Kv1.3. ΔC cannot interact with MAGUK-s and is not enriched at the IS, whereas cells expressing the non-conducting mutant are depolarized. Here, we found that in standalone cells, mobility of ΔC increased relative to the WT, likely due to abrogation of interactions, whereas mobility of the non-conducting mutant decreased, similar to our previous observations on other membrane proteins in depolarized cells. At the IS formed with Raji B-cells, mobility of WT and non-conducting channels, unlike ΔC, was lower than outside the IS. The Kv1.3 variants possessing an intact C-terminus had lower mobility in standalone cells than in IS-engaged cells. This may be related to the observed segregation of F-actin into a ring-like structure at the periphery of the IS, leaving much of the cell almost void of F-actin. Upon depolarizing treatment, mobility of WT and ΔC channels decreased both in standalone and IS-engaged cells, contrary to non-conducting channels, which themselves caused depolarization. Our results support that Kv1.3 is enriched at the IS via its C-terminal region regardless of conductivity, and that depolarization decreases channel mobility.
Keywords: Kv1.3 channel; T cell; fluorescence correlation spectroscopy; immunological synapse; live cell; membrane depolarization; mobility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Functional consequences of Kv1.3 ion channel rearrangement into the immunological synapse.Immunol Lett. 2009 Jun 30;125(1):15-21. doi: 10.1016/j.imlet.2009.05.004. Epub 2009 May 27. Immunol Lett. 2009. PMID: 19477198
-
The C-terminus SH3-binding domain of Kv1.3 is required for the actin-mediated immobilization of the channel via cortactin.Mol Biol Cell. 2015 May 1;26(9):1640-51. doi: 10.1091/mbc.E14-07-1195. Epub 2015 Mar 4. Mol Biol Cell. 2015. PMID: 25739456 Free PMC article.
-
Localization of Kv1.3 channels in the immunological synapse modulates the calcium response to antigen stimulation in T lymphocytes.J Immunol. 2009 Nov 15;183(10):6296-302. doi: 10.4049/jimmunol.0900613. Epub 2009 Oct 19. J Immunol. 2009. PMID: 19841189 Free PMC article.
-
The secret life of ion channels: Kv1.3 potassium channels and proliferation.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C27-C42. doi: 10.1152/ajpcell.00136.2017. Epub 2017 Sep 20. Am J Physiol Cell Physiol. 2018. PMID: 28931540 Review.
-
Voltage-Gated Potassium Channels Kv1.3--Potentially New Molecular Target in Cancer Diagnostics and Therapy.Adv Clin Exp Med. 2015 May-Jun;24(3):517-24. doi: 10.17219/acem/22339. Adv Clin Exp Med. 2015. PMID: 26467143 Review.
Cited by
-
Dynamics and spatial organization of Kv1.3 at the immunological synapse of human CD4+ T cells.Biophys J. 2024 Aug 6;123(15):2271-2281. doi: 10.1016/j.bpj.2023.08.011. Epub 2023 Aug 18. Biophys J. 2024. PMID: 37596785 Free PMC article.
-
Identification of novel Kv1.3 channel-interacting proteins using proximity labelling in T-cells.bioRxiv [Preprint]. 2025 Jan 18:2025.01.16.633279. doi: 10.1101/2025.01.16.633279. bioRxiv. 2025. PMID: 39868101 Free PMC article. Preprint.
References
-
- Beeton C., Wulff H., Singh S., Botsko S., Crossley G., Gutman G.A., Cahalan M.D., Pennington M., Chandy K.G. A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes. J. Biol. Chem. 2003;278:9928–9937. doi: 10.1074/jbc.M212868200. - DOI - PubMed
-
- Beeton C., Wulff H., Standifer N.E., Azam P., Mullen K.M., Pennington M.W., Kolski-Andreaco A., Wei E., Grino A., Counts D.R., et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

