The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders
- PMID: 35328751
- PMCID: PMC8955937
- DOI: 10.3390/ijms23063326
The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders
Abstract
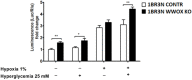
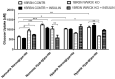
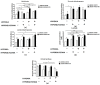
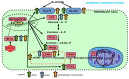
Recent reports indicate that the hypoxia-induced factor (HIF1α) and the Warburg effect play an initiating role in glucotoxicity, which underlies disorders in metabolic diseases. WWOX has been identified as a HIF1α regulator. WWOX downregulation leads to an increased expression of HIF1α target genes encoding glucose transporters and glycolysis' enzymes. It has been proven in the normoglycemic mice cells and in gestational diabetes patients. The aim of the study was to determine WWOX's role in glucose metabolism regulation in hyperglycemia and hypoxia to confirm its importance in the development of metabolic disorders. For this purpose, the WWOX gene was silenced in human normal fibroblasts, and then cells were cultured under different sugar and oxygen levels. Thereafter, it was investigated how WWOX silencing alters the genes and proteins expression profile of glucose transporters and glycolysis pathway enzymes, and their activity. In normoxia normoglycemia, higher glycolysis genes expression, their activity, and the lactate concentration were observed in WWOX KO fibroblasts in comparison to control cells. In normoxia hyperglycemia, it was observed a decrease of insulin-dependent glucose uptake and a further increase of lactate. It likely intensifies hyperglycemia condition, which deepen the glucose toxic effect. Then, in hypoxia hyperglycemia, WWOX KO caused weaker glucose uptake and elevated lactate production. In conclusion, the WWOX/HIF1A axis downregulation alters glucose metabolism and probably predispose to metabolic disorders.
Keywords: WW domain-containing oxidoreductase (WWOX); glycolysis; hypoxia-inducible factor 1α (HIF1α); metabolic disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





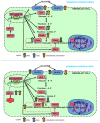
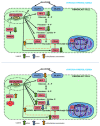
Similar articles
-
WWOX and metabolic regulation in normal and pathological conditions.J Mol Med (Berl). 2022 Dec;100(12):1691-1702. doi: 10.1007/s00109-022-02265-5. Epub 2022 Oct 22. J Mol Med (Berl). 2022. PMID: 36271927 Free PMC article. Review.
-
Tumor suppressor WWOX regulates glucose metabolism via HIF1α modulation.Cell Death Differ. 2014 Nov;21(11):1805-14. doi: 10.1038/cdd.2014.95. Epub 2014 Jul 11. Cell Death Differ. 2014. PMID: 25012504 Free PMC article.
-
Identification of a novel association for the WWOX/HIF1A axis with gestational diabetes mellitus (GDM).PeerJ. 2021 Jan 14;9:e10604. doi: 10.7717/peerj.10604. eCollection 2021. PeerJ. 2021. PMID: 33520443 Free PMC article.
-
WWOX attenuates the progression of gallbladder cancer by suppressing cellular glycolysis through the modulation of the P73/HIF-1α signaling pathway.Tissue Cell. 2025 Aug;95:102885. doi: 10.1016/j.tice.2025.102885. Epub 2025 Apr 2. Tissue Cell. 2025. PMID: 40198927
-
The tumor suppressor WW domain-containing oxidoreductase modulates cell metabolism.Exp Biol Med (Maywood). 2015 Mar;240(3):345-50. doi: 10.1177/1535370214561956. Epub 2014 Dec 8. Exp Biol Med (Maywood). 2015. PMID: 25491415 Free PMC article. Review.
Cited by
-
Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration.Int J Mol Sci. 2024 Mar 20;25(6):3507. doi: 10.3390/ijms25063507. Int J Mol Sci. 2024. PMID: 38542478 Free PMC article. Review.
-
Twenty-five years of WWOX insight in cancer: a treasure trove of knowledge.Funct Integr Genomics. 2025 May 6;25(1):100. doi: 10.1007/s10142-025-01601-5. Funct Integr Genomics. 2025. PMID: 40327201 Free PMC article. Review.
-
WWOX and metabolic regulation in normal and pathological conditions.J Mol Med (Berl). 2022 Dec;100(12):1691-1702. doi: 10.1007/s00109-022-02265-5. Epub 2022 Oct 22. J Mol Med (Berl). 2022. PMID: 36271927 Free PMC article. Review.
-
Mechanistic Investigation of WWOX Function in NF-kB-Induced Skin Inflammation in Psoriasis.Int J Mol Sci. 2023 Dec 21;25(1):167. doi: 10.3390/ijms25010167. Int J Mol Sci. 2023. PMID: 38203337 Free PMC article.
-
WWOX tuning of oleic acid signaling orchestrates immunosuppressive macrophage polarization and sensitizes hepatocellular carcinoma to immunotherapy.J Immunother Cancer. 2024 Nov 5;12(11):e010422. doi: 10.1136/jitc-2024-010422. J Immunother Cancer. 2024. PMID: 39500530 Free PMC article.
References
-
- Gao W., Ferguson G., Connell P., Walshe T., Murphy R., Birney Y.A., O’Brien C., Cahill P.A. High Glucose Concentrations Alter Hypoxia-Induced Control of Vascular Smooth Muscle Cell Growth via a HIF-1α-Dependent Pathway. J. Mol. Cell. Cardiol. 2007;42:609–619. doi: 10.1016/j.yjmcc.2006.12.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

