The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis
- PMID: 35328793
- PMCID: PMC8952310
- DOI: 10.3390/ijms23063370
The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis
Abstract
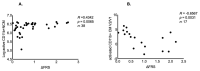
Monocytes expressing the inflammation suppressing active CD11b, a beta2 integrin, may regulate neuroinflammation and modify clinical outcomes in amyotrophic lateral sclerosis (ALS). In this single site, retrospective study, peripheral blood mononuclear cells from 38 individuals living with ALS and 20 non-neurological controls (NNC) were investigated using flow cytometry to study active CD11b integrin classical (CM), intermediate (IM) and non-classical (NCM) monocytes during ALS progression. Seventeen ALS participants were sampled at the baseline (V1) and at two additional time points (V2 and V3) for longitudinal analysis. Active CD11b+ CM frequencies increased steeply between the baseline and V3 (ANOVA repeated measurement, p < 0.001), and the V2/V1 ratio negatively correlated with the disease progression rate, similar to higher frequencies of active CD11b+ NCM at the baseline (R = −0.6567; p = 0.0031 and R = 0.3862; p = 0.0168, respectively). CD11b NCM, clinical covariates and neurofilament light-chain plasma concentration at the baseline predicted shorter survival in a multivariable and univariate analysis (CD11b NCM—HR: 1.05, CI: 1.01−1.11, p = 0.013. Log rank: above median: 43 months and below median: 21.22 months; p = 0.0022). Blood samples with the highest frequencies of active CD11b+ IM and NCM contained the lowest concentrations of soluble CD11b. Our preliminary data suggest that the levels of active CD11b+ monocytes and NCM in the blood predict different clinical outcomes in ALS.
Keywords: CD11b; amyotrophic lateral sclerosis; beta2 integrin; monocytes.
Conflict of interest statement
O. Yildiz, J. Schroth, V. Lombardi, V. Pucino, Y. Bobeva, Ping K. Yip, K. Schmierer, C. Mauro, T. Tree, and S. M. Henson report no disclosures related to submitted work. A. Malaspina has acted as consultant and received personal fees from Roche and Pfizer. He has worked as site investigator and Work-Package Lead on clinical trials funded by EU2020. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures



References
-
- Bede P., Bokde A., Elamin M., Byrne S., McLaughlin R.L., Jordan N., Hampel H., Gallagher L., Lynch C., Fagan A.J., et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry. 2012;84:766–773. doi: 10.1136/jnnp-2012-302674. - DOI - PubMed
-
- Mantovani S., Garbelli S., Pasini A., Alimonti D., Perotti C., Melazzini M., Bendotti C., Mora G. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J. Neuroimmunol. 2009;210:73–79. doi: 10.1016/j.jneuroim.2009.02.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

