Rapid Identification of Pollen- and Anther-Specific Genes in Response to High-Temperature Stress Based on Transcriptome Profiling Analysis in Cotton
- PMID: 35328797
- PMCID: PMC8954629
- DOI: 10.3390/ijms23063378
Rapid Identification of Pollen- and Anther-Specific Genes in Response to High-Temperature Stress Based on Transcriptome Profiling Analysis in Cotton
Abstract
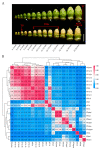
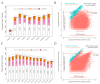
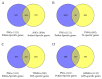
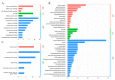
Anther indehiscence and pollen sterility caused by high temperature (HT) stress have become a major problem that decreases the yield of cotton. Pollen- and anther-specific genes play a critical role in the process of male reproduction and the response to HT stress. In order to identify pollen-specific genes that respond to HT stress, a comparative transcriptome profiling analysis was performed in the pollen and anthers of Gossypium hirsutum HT-sensitive Line H05 against other tissue types under normal temperature (NT) conditions, and the analysis of a differentially expressed gene was conducted in the pollen of H05 under NT and HT conditions. In total, we identified 1111 pollen-specific genes (PSGs), 1066 anther-specific genes (ASGs), and 833 pollen differentially expressed genes (DEGs). Moreover, we found that the late stage of anther included more anther- and pollen-specific genes (APSGs). Stress-related cis-regulatory elements (CREs) and hormone-responsive CREs are enriched in the promoters of APSGs, suggesting that APSGs may respond to HT stress. However, 833 pollen DEGs had only 10 common genes with 1111 PSGs, indicating that PSGs are mainly involved in the processes of pollen development and do not respond to HT stress. Promoters of these 10 common genes are enriched for stress-related CREs and MeJA-responsive CREs, suggesting that these 10 common genes are involved in the process of pollen development while responding to HT stress. This study provides a pathway for rapidly identifying cotton pollen-specific genes that respond to HT stress.
Keywords: Gossypium hirsutum; high-temperature stress; male-sterility; pollen-specific genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lobell D., Field C.B. Global scale climate–crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007;2:014002. doi: 10.1088/1748-9326/2/1/014002. - DOI
-
- Schauberger B., Archontoulis S., Arneth A., Balkovic J., Ciais P., Deryng D., Elliott J., Folberth C., Khabarov N., Müller C., et al. Consistent negative response of US crops to high temperatures in observations and crop models. Nat. Commun. 2017;8:13931. doi: 10.1038/ncomms13931. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- 2021ZKPY019; 2016YFD0101402/Fundamental Research Funds for the Central Universities; National Key Research and Development Program of China
- 2020172-2/Platform Construction of Genetic Improvement and Molecular Design Breeding for Xinjiang Island Cotton
- 32072024/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials

