Two Triacylglycerol Lipases Are Negative Regulators of Chilling Stress Tolerance in Arabidopsis
- PMID: 35328798
- PMCID: PMC8950723
- DOI: 10.3390/ijms23063380
Two Triacylglycerol Lipases Are Negative Regulators of Chilling Stress Tolerance in Arabidopsis
Abstract
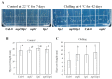
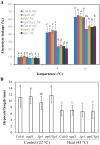
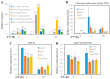
Cold stress is one of the abiotic stress conditions that severely limit plant growth and development and productivity. Triacylglycerol lipases are important metabolic enzymes for the catabolism of triacylglycerols and, therefore, play important roles in cellular activities including seed germination and early seedling establishment. However, whether they play a role in cold stress responses remains unknown. In this study, we characterized two Arabidopsis triacylglycerol lipases, MPL1 and LIP1 and defined their role in cold stress. The expression of MPL1 and LIP1 is reduced by cold stress, suggesting that they may be negative factors related to cold stress. Indeed, we found that loss-of-function of MPL1 and LIP1 resulted in increased cold tolerance and that the mpl1lip1 double mutant displayed an additive effect on cold tolerance. We performed RNA-seq analysis to reveal the global effect of the mpl1 and lip1 mutations on gene expression under cold stress. The mpl1 mutation had a small effect on gene expression under both under control and cold stress conditions whereas the lip1 mutation caused a much stronger effect on gene expression under control and cold stress conditions. The mpl1lip1 double mutant had a moderate effect on gene expression under control and cold stress conditions. Together, our results indicate that MPL1 and LIP1 triacylglycerol lipases are negative regulators of cold tolerance without any side effects on growth in Arabidopsis and that they might be ideal candidates for breeding cold-tolerant crops through genome editing technology.
Keywords: Arabidopsis; chilling stress tolerance; cold stress; triacylglycerol lipases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antibiosis against the green peach aphid requires the Arabidopsis thaliana MYZUS PERSICAE-INDUCED LIPASE1 gene.Plant J. 2010 Dec;64(5):800-11. doi: 10.1111/j.1365-313X.2010.04378.x. Epub 2010 Oct 26. Plant J. 2010. PMID: 21105927
-
Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis.Plant Physiol. 2011 Oct;157(2):866-75. doi: 10.1104/pp.111.181784. Epub 2011 Aug 8. Plant Physiol. 2011. PMID: 21825108 Free PMC article.
-
Two Groups of Thellungiella salsuginea RAVs Exhibit Distinct Responses and Sensitivity to Salt and ABA in Transgenic Arabidopsis.PLoS One. 2016 Apr 19;11(4):e0153517. doi: 10.1371/journal.pone.0153517. eCollection 2016. PLoS One. 2016. PMID: 27093611 Free PMC article.
-
Storage oil hydrolysis during early seedling growth.Plant Physiol Biochem. 2009 Jun;47(6):485-90. doi: 10.1016/j.plaphy.2008.12.005. Epub 2008 Dec 16. Plant Physiol Biochem. 2009. PMID: 19136267 Review.
-
Cell Membrane Features as Potential Breeding Targets to Improve Cold Germination Ability of Seeds.Plants (Basel). 2022 Dec 6;11(23):3400. doi: 10.3390/plants11233400. Plants (Basel). 2022. PMID: 36501439 Free PMC article. Review.
Cited by
-
Enhancement of Apple Stress Resistance via Proline Elevation by Sugar Substitutes.Int J Mol Sci. 2024 Sep 2;25(17):9548. doi: 10.3390/ijms25179548. Int J Mol Sci. 2024. PMID: 39273495 Free PMC article.
-
Ammonia borane positively regulates cold tolerance in Brassica napus via hydrogen sulfide signaling.BMC Plant Biol. 2022 Dec 14;22(1):585. doi: 10.1186/s12870-022-03973-3. BMC Plant Biol. 2022. PMID: 36517759 Free PMC article.
-
Molecular signatures that translate across omics layers and crops under high aluminium and low phosphorus stress facilitate the identification of reliable molecular targets for genotyping in lentil.Funct Integr Genomics. 2025 Mar 5;25(1):52. doi: 10.1007/s10142-025-01542-z. Funct Integr Genomics. 2025. PMID: 40042647
-
Comparative Transcriptomics and Metabolites Analysis of Two Closely Related Euphorbia Species Reveal Environmental Adaptation Mechanism and Active Ingredients Difference.Front Plant Sci. 2022 May 31;13:905275. doi: 10.3389/fpls.2022.905275. eCollection 2022. Front Plant Sci. 2022. PMID: 35712557 Free PMC article.
References
-
- Stockinger E.J., Gilmour S.J., Thomashow M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. - DOI - PMC - PubMed
-
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

