Two Male-Specific Antimicrobial Peptides SCY2 and Scyreprocin as Crucial Molecules Participated in the Sperm Acrosome Reaction of Mud Crab Scylla paramamosain
- PMID: 35328805
- PMCID: PMC8952799
- DOI: 10.3390/ijms23063373
Two Male-Specific Antimicrobial Peptides SCY2 and Scyreprocin as Crucial Molecules Participated in the Sperm Acrosome Reaction of Mud Crab Scylla paramamosain
Abstract
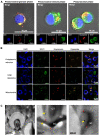
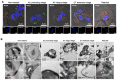
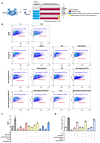
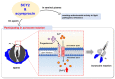
Antimicrobial peptides (AMPs) identified in the reproductive system of animals have been widely studied for their antimicrobial activity, but only a few studies have focused on their physiological roles. Our previous studies have revealed the in vitro antimicrobial activity of two male gonadal AMPs, SCY2 and scyreprocin, from mud crab Scylla paramamosain. Their physiological functions, however, remain a mystery. In this study, the two AMPs were found co-localized on the sperm apical cap. Meanwhile, progesterone was confirmed to induce acrosome reaction (AR) of mud crab sperm in vitro, which intrigued us to explore the roles of the AMPs and progesterone in AR. Results showed that the specific antibody blockade of scyreprocin inhibited the progesterone-induced AR without affecting intracellular Ca2+ homeostasis, while the blockade of SCY2 hindered the influx of Ca2+. We further showed that SCY2 could directly bind to Ca2+. Moreover, progesterone failed to induce AR when either scyreprocin or SCY2 function was deprived. Taken together, scyreprocin and SCY2 played a dual role in reproductive immunity and sperm AR. To our knowledge, this is the first report on the direct involvement of AMPs in sperm AR, which would expand the current understanding of the roles of AMPs in reproduction.
Keywords: SCY2; acrosome reaction; antimicrobial peptide (AMP); fertilization; invertebrate; progesterone; scyreprocin; sperm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
A New Gene SCY3 Homologous to Scygonadin Showing Antibacterial Activity and a Potential Role in the Sperm Acrosome Reaction of Scylla paramamosain.Int J Mol Sci. 2023 Mar 16;24(6):5689. doi: 10.3390/ijms24065689. Int J Mol Sci. 2023. PMID: 36982761 Free PMC article.
-
A Novel Antimicrobial Peptide Scyreprocin From Mud Crab Scylla paramamosain Showing Potent Antifungal and Anti-biofilm Activity.Front Microbiol. 2020 Jul 24;11:1589. doi: 10.3389/fmicb.2020.01589. eCollection 2020. Front Microbiol. 2020. PMID: 32849331 Free PMC article.
-
A new antimicrobial peptide SCY2 identified in Scylla Paramamosain exerting a potential role of reproductive immunity.Fish Shellfish Immunol. 2016 Apr;51:251-262. doi: 10.1016/j.fsi.2016.02.022. Epub 2016 Feb 18. Fish Shellfish Immunol. 2016. PMID: 26911409
-
Progesterone, spermatozoa and reproduction: An updated review.Mol Cell Endocrinol. 2020 Oct 1;516:110952. doi: 10.1016/j.mce.2020.110952. Epub 2020 Jul 24. Mol Cell Endocrinol. 2020. PMID: 32712385 Review.
-
Acrosome reaction in the cumulus oophorus revisited: involvement of a novel sperm-released factor NYD-SP8.Protein Cell. 2011 Feb;2(2):92-8. doi: 10.1007/s13238-011-1022-5. Epub 2011 Mar 5. Protein Cell. 2011. PMID: 21380641 Free PMC article. Review.
Cited by
-
Antiviral and antibacterial peptides: Mechanisms of action.Heliyon. 2024 Nov 5;10(22):e40121. doi: 10.1016/j.heliyon.2024.e40121. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39748995 Free PMC article. Review.
-
High-resolution chromosome-level genome of Scylla paramamosain provides molecular insights into adaptive evolution in crabs.BMC Biol. 2024 Nov 7;22(1):255. doi: 10.1186/s12915-024-02054-1. BMC Biol. 2024. PMID: 39511558 Free PMC article.
-
The Anticancer Activity Conferred by the Mud Crab Antimicrobial Peptide Scyreprocin through Apoptosis and Membrane Disruption.Int J Mol Sci. 2022 May 14;23(10):5500. doi: 10.3390/ijms23105500. Int J Mol Sci. 2022. PMID: 35628312 Free PMC article.
-
Frontiers in Antimicrobial Biomaterials.Int J Mol Sci. 2022 Aug 19;23(16):9377. doi: 10.3390/ijms23169377. Int J Mol Sci. 2022. PMID: 36012640 Free PMC article.
-
A New Gene SCY3 Homologous to Scygonadin Showing Antibacterial Activity and a Potential Role in the Sperm Acrosome Reaction of Scylla paramamosain.Int J Mol Sci. 2023 Mar 16;24(6):5689. doi: 10.3390/ijms24065689. Int J Mol Sci. 2023. PMID: 36982761 Free PMC article.
References
-
- Stival C., Puga Molina L.D.C., Paudel B., Buffone M., Visconti P., Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Sperm Acrosome Biog. Funct. Dur. Fertil. 2016;220:93–106. - PubMed
-
- Dan J.C. Studies on the acrosome. I. Reaction to egg-water and other stimuli. Biol. Bull. 1952;103:54–66. doi: 10.2307/1538405. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

