Swelling, Protein Adsorption, and Biocompatibility In Vitro of Gel Beads Prepared from Pectin of Hogweed Heracleum sosnówskyi Manden in Comparison with Gel Beads from Apple Pectin
- PMID: 35328806
- PMCID: PMC8954847
- DOI: 10.3390/ijms23063388
Swelling, Protein Adsorption, and Biocompatibility In Vitro of Gel Beads Prepared from Pectin of Hogweed Heracleum sosnówskyi Manden in Comparison with Gel Beads from Apple Pectin
Abstract
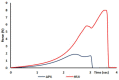
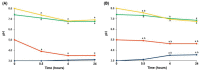
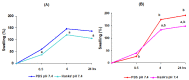
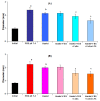
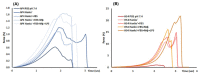
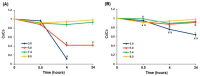
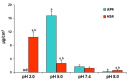
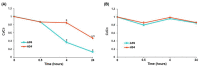
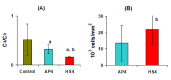
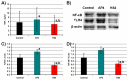
The study aims to develop gel beads with improved functional properties and biocompatibility from hogweed (HS) pectin. HS4 and AP4 gel beads were prepared from the HS pectin and apple pectin (AP) using gelling with calcium ions. HS4 and AP4 gel beads swelled in PBS in dependence on pH. The swelling degree of HS4 and AP4 gel beads was 191 and 136%, respectively, in PBS at pH 7.4. The hardness of HS4 and AP4 gel beads reduced 8.2 and 60 times, respectively, compared with the initial value after 24 h incubation. Both pectin gel beads swelled less in Hanks' solution than in PBS and swelled less in Hanks' solution containing peritoneal macrophages than in cell-free Hanks' solution. Serum protein adsorption by HS4 and AP4 gel beads was 118 ± 44 and 196 ± 68 μg/cm2 after 24 h of incubation. Both pectin gel beads demonstrated low rates of hemolysis and complement activation. However, HS4 gel beads inhibited the LPS-stimulated secretion of TNF-α and the expression of TLR4 and NF-κB by macrophages, whereas AP4 gel beads stimulated the inflammatory response of macrophages. HS4 gel beads adsorbed 1.3 times more LPS and adhered to 1.6 times more macrophages than AP4 gel beads. Thus, HS pectin gel has advantages over AP gel concerning swelling behavior, protein adsorption, and biocompatibility.
Keywords: TNF-α; apple pectin; complement activation; gel beads; hemolysis; hogweed pectin; peritoneal macrophages; protein adsorption; swelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Swelling, Protein Adsorption, and Biocompatibility of Pectin-Chitosan Hydrogels.Gels. 2024 Jul 17;10(7):472. doi: 10.3390/gels10070472. Gels. 2024. PMID: 39057495 Free PMC article.
-
Effect of Cross-Linking Cations on In Vitro Biocompatibility of Apple Pectin Gel Beads.Int J Mol Sci. 2022 Nov 26;23(23):14789. doi: 10.3390/ijms232314789. Int J Mol Sci. 2022. PMID: 36499122 Free PMC article.
-
Characterization and Biocompatibility Properties In Vitro of Gel Beads Based on the Pectin and κ-Carrageenan.Mar Drugs. 2022 Jan 22;20(2):94. doi: 10.3390/md20020094. Mar Drugs. 2022. PMID: 35200624 Free PMC article.
-
[Sosnowsky's hogweed - toxicology and threat to health].Pol Merkur Lekarski. 2016 Sep 29;41(243):165-168. Pol Merkur Lekarski. 2016. PMID: 27755521 Review. Polish.
-
Chemistry and uses of pectin--a review.Crit Rev Food Sci Nutr. 1997 Feb;37(1):47-73. doi: 10.1080/10408399709527767. Crit Rev Food Sci Nutr. 1997. PMID: 9067088 Review.
Cited by
-
Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel.Gels. 2023 Mar 14;9(3):225. doi: 10.3390/gels9030225. Gels. 2023. PMID: 36975674 Free PMC article.
-
Effect of Chitosan on Rheological, Mechanical, and Adhesive Properties of Pectin-Calcium Gel.Mar Drugs. 2023 Jun 25;21(7):375. doi: 10.3390/md21070375. Mar Drugs. 2023. PMID: 37504906 Free PMC article.
-
Texture Perception and Chewing of Agar Gel by People with Different Sensitivity to Hardness.Gels. 2024 Dec 26;11(1):5. doi: 10.3390/gels11010005. Gels. 2024. PMID: 39851976 Free PMC article.
-
Swelling, Protein Adsorption, and Biocompatibility of Pectin-Chitosan Hydrogels.Gels. 2024 Jul 17;10(7):472. doi: 10.3390/gels10070472. Gels. 2024. PMID: 39057495 Free PMC article.
-
Effect of Cross-Linking Cations on In Vitro Biocompatibility of Apple Pectin Gel Beads.Int J Mol Sci. 2022 Nov 26;23(23):14789. doi: 10.3390/ijms232314789. Int J Mol Sci. 2022. PMID: 36499122 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

