Brain Cholesterol Biosynthetic Pathway Is Altered in a Preclinical Model of Fragile X Syndrome
- PMID: 35328827
- PMCID: PMC8955806
- DOI: 10.3390/ijms23063408
Brain Cholesterol Biosynthetic Pathway Is Altered in a Preclinical Model of Fragile X Syndrome
Abstract
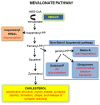
Fragile X Syndrome (FXS) is the most frequent form of inherited X-linked pathology, associated with an intellectual and developmental disability, and currently considered the first monogenic cause of autism spectrum disorder (ASD). Low levels of total cholesterol reported in the serum of FXS patients, and evidence that FMRP targets a subset of mRNAs encoding proteins of lipid synthesis and transport suggests that the cholesterol metabolism impairments could be involved in FXS. Thus, the aim of the presented work was to investigate the modulations of the cholesterol biosynthetic pathway and its end-products in a recently developed Fmr1-Δexon 8 rat model of FXS. Here, we show that this experimental model mimics what is found in FXS patients, exhibiting a lower serum cholesterol content, accompanied by a reduction in food intake and body weight compared to WT animals. Moreover, alterations of proteins committed to cholesterol synthesis and uptake have been observed in the amygdala, prefrontal cortex and nucleus accumbens. Interestingly, the end-products show a brain region-dependent modulation in Fmr1-Δexon 8 rats. Overall, our results demonstrate that the cholesterol biosynthetic pathway is altered in some brain regions of this preclinical model of FXS. This finding has relevance for future studies to delve deeper into the involvement of this metabolic process in FXS, and thus its possible role as a therapeutic target.
Keywords: 3-Hydroxy 3-methylglutaryl Coenzyme A reductase; Fmr1-Δexon 8 rat; Fragile X Syndrome; brain; cholesterol; liver; low-density lipoprotein receptor; plasma; prenylated proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Musetti A., Manari T., Dioni B., Raffin C., Bravo G., Mariani R., Esposito G., Dimitriou D., Plazzi G., Franceschini C., et al. Parental Quality of Life and Involvement in Intervention for Children or Adolescents with Autism Spectrum Disorders: A Systematic Review. J. Pers. Med. 2021;11:894. doi: 10.3390/jpm11090894. - DOI - PMC - PubMed
-
- Prieto M., Folci A., Poupon G., Schiavi S., Buzzelli V., Pronot M., François U., Pousinha P., Lattuada N., Abelanet S., et al. Missense Mutation of Fmr1 Results in Impaired AMPAR-Mediated Plasticity and Socio-Cognitive Deficits in Mice. Nat. Commun. 2021;12:1557. doi: 10.1038/s41467-021-21820-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

