Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia-Reperfusion
- PMID: 35328832
- PMCID: PMC8955307
- DOI: 10.3390/ijms23063411
Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia-Reperfusion
Abstract
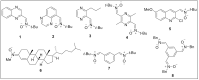
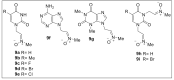
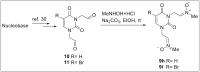
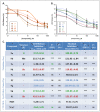
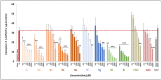
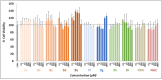
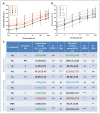
Herein, we report the synthesis, antioxidant, and neuroprotective properties of some nucleobase-derived nitrones named 9a-i. The neuroprotective properties of nitrones, 9a-i, were measured against an oxygen-glucose-deprivation in vitro ischemia model using human neuroblastoma SH-SY5Y cells. Our results indicate that nitrones, 9a-i, have better neuroprotective and antioxidant properties than α-phenyl-N-tert-butylnitrone (PBN) and are similar to N-acetyl-L-cysteine (NAC), a well-known antioxidant and neuroprotective agent. The nitrones with the highest neuroprotective capacity were those containing purine nucleobases (nitrones 9f, g, B = adenine, theophylline), followed by nitrones with pyrimidine nucleobases with H or F substituents at the C5 position (nitrones 9a, c). All of these possess EC50 values in the range of 1-6 μM and maximal activities higher than 100%. However, the introduction of a methyl substituent (nitrone 9b, B = thymine) or hard halogen substituents such as Br and Cl (nitrones 9d, e, B = 5-Br and 5-Cl uracil, respectively) worsens the neuroprotective activity of the nitrone with uracil as the nucleobase (9a). The effects on overall metabolic cell capacity were confirmed by results on the high anti-necrotic (EC50's ≈ 2-4 μM) and antioxidant (EC50's ≈ 0.4-3.5 μM) activities of these compounds on superoxide radical production. In general, all tested nitrones were excellent inhibitors of superoxide radical production in cultured neuroblastoma cells, as well as potent hydroxyl radical scavengers that inhibit in vitro lipid peroxidation, particularly, 9c, f, g, presenting the highest lipoxygenase inhibitory activity among the tested nitrones. Finally, the introduction of two nitrone groups at 9a and 9d (bis-nitronas 9g, i) did not show better neuroprotective effects than their precursor mono-nitrones. These results led us to propose nitrones containing purine (9f, g) and pyrimidine (9a, c) nucleobases as potential therapeutic agents for the treatment of cerebral ischemia and/or neurodegenerative diseases, leading us to further investigate their effects using in vivo models of these pathologies.
Keywords: antioxidants; apoptosis; brain ischemia; necrosis; neuroprotection; nucleobase-derived nitrones; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis, Neuroprotection, and Antioxidant Activity of 1,1'-Biphenylnitrones as α-Phenyl-N-tert-butylnitrone Analogues in In Vitro Ischemia Models.Molecules. 2021 Feb 20;26(4):1127. doi: 10.3390/molecules26041127. Molecules. 2021. PMID: 33672652 Free PMC article.
-
Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived HomoBisNitrones in in vitro and in vivo ischemia models.Sci Rep. 2020 Aug 25;10(1):14150. doi: 10.1038/s41598-020-70690-y. Sci Rep. 2020. PMID: 32843666 Free PMC article.
-
Homo-Tris-Nitrones Derived from α-Phenyl-N-tert-butylnitrone: Synthesis, Neuroprotection and Antioxidant Properties.Int J Mol Sci. 2020 Oct 26;21(21):7949. doi: 10.3390/ijms21217949. Int J Mol Sci. 2020. PMID: 33114714 Free PMC article.
-
Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059.Pharmacol Ther. 2003 Dec;100(3):195-214. doi: 10.1016/j.pharmthera.2003.07.003. Pharmacol Ther. 2003. PMID: 14652110 Review.
-
Diagnostic and therapeutic applications of azulenyl nitrone spin traps.Cell Mol Life Sci. 1999 Nov 15;56(7-8):626-33. doi: 10.1007/s000180050457. Cell Mol Life Sci. 1999. PMID: 11212310 Free PMC article. Review.
Cited by
-
Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones.Antioxidants (Basel). 2022 Dec 24;12(1):36. doi: 10.3390/antiox12010036. Antioxidants (Basel). 2022. PMID: 36670898 Free PMC article.
-
Phosphorylated Nitrones-Synthesis and Applications.Molecules. 2025 Mar 16;30(6):1333. doi: 10.3390/molecules30061333. Molecules. 2025. PMID: 40142107 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

