Cost-Effectiveness of Routine Third Trimester Ultrasound Screening for Fetal Growth Restriction Compared to Care as Usual in Low-Risk Pregnancies: A Pragmatic Nationwide Stepped-Wedge Cluster-Randomized Trial in The Netherlands (the IRIS Study)
- PMID: 35329004
- PMCID: PMC8955489
- DOI: 10.3390/ijerph19063312
Cost-Effectiveness of Routine Third Trimester Ultrasound Screening for Fetal Growth Restriction Compared to Care as Usual in Low-Risk Pregnancies: A Pragmatic Nationwide Stepped-Wedge Cluster-Randomized Trial in The Netherlands (the IRIS Study)
Abstract
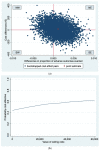
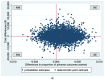
Routine third trimester ultrasonography is increasingly used to screen for fetal growth restriction. However, evidence regarding its cost-effectiveness is lacking. We aimed to evaluate the cost-effectiveness of routine third trimester ultrasonography to reduce adverse perinatal outcomes compared to usual care (selective ultrasonography). An economic evaluation alongside a stepped-wedge cluster-randomized trial was conducted. Via 60 midwifery practices 12,974 Dutch women aged ≥16 years with low-risk pregnancies were enrolled at 22.8 (SD = 2.4) weeks' gestation. All practices provided usual care. At 3, 7, and 10 months a third of the practices were randomized to the intervention strategy providing routine ultrasonography at 28-30 and 34-36 weeks' gestation and usual care. The primary clinical outcome was a dichotomous composite measure of 12 severe adverse perinatal outcomes (SAPO) up to one week postpartum. Information on perinatal care and societal costs was derived from Netherlands Perinatal Registry, hospital records and a survey. Cost-effectiveness analyses revealed no significant differences in SAPO and healthcare and societal costs between the intervention strategy (n = 7026) and usual care (n = 5948). Cost-effectiveness acceptability curves showed that the probability of cost-effectiveness was never higher than 0.6 for all possible ceiling ratios. Adding routine third trimester ultrasonography to usual care is not cost-effective in reducing SAPO.
Keywords: cluster-randomized trial; economic evaluation; routine third trimester ultrasonography; severe adverse perinatal outcome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effectiveness of routine third trimester ultrasonography to reduce adverse perinatal outcomes in low risk pregnancy (the IRIS study): nationwide, pragmatic, multicentre, stepped wedge cluster randomised trial.BMJ. 2019 Oct 15;367:l5517. doi: 10.1136/bmj.l5517. BMJ. 2019. PMID: 31615781 Free PMC article. Clinical Trial.
-
Effectiveness and cost-effectiveness of routine third trimester ultrasound screening for intrauterine growth restriction: study protocol of a nationwide stepped wedge cluster-randomized trial in The Netherlands (The IRIS Study).BMC Pregnancy Childbirth. 2016 Oct 13;16(1):310. doi: 10.1186/s12884-016-1104-8. BMC Pregnancy Childbirth. 2016. PMID: 27737654 Free PMC article. Clinical Trial.
-
Prediction of large-for-gestational-age neonate by routine third-trimester ultrasound.Ultrasound Obstet Gynecol. 2019 Sep;54(3):326-333. doi: 10.1002/uog.20377. Epub 2019 Jul 23. Ultrasound Obstet Gynecol. 2019. PMID: 31236963
-
Ultrasound in twin pregnancies.J Obstet Gynaecol Can. 2011 Jun;33(6):643-656. doi: 10.1016/S1701-2163(16)34916-7. J Obstet Gynaecol Can. 2011. PMID: 21846456 Review.
-
Third-trimester uterine artery Doppler for prediction of adverse outcome in late small-for-gestational-age fetuses: systematic review and meta-analysis.Ultrasound Obstet Gynecol. 2020 May;55(5):575-585. doi: 10.1002/uog.21940. Ultrasound Obstet Gynecol. 2020. PMID: 31785172
Cited by
-
Meta-analysis comparing different ultrasound detection methods to accurately assess wound healing and scar formation after caesarean section.Int Wound J. 2024 Apr;21(4):e14837. doi: 10.1111/iwj.14837. Int Wound J. 2024. Retraction in: Int Wound J. 2024 Dec;21(12):e70161. doi: 10.1111/iwj.70161. PMID: 38629613 Free PMC article. Retracted.
-
Research protocol - Evaluating data quality in the Netherlands Perinatal Registry (Perined): A data comparison study using hospital records from the IUGR Risk Selection (IRIS) study.F1000Res. 2025 Jan 9;13:686. doi: 10.12688/f1000research.150160.2. eCollection 2024. F1000Res. 2025. PMID: 39949967 Free PMC article.
-
Perinatal and Neonatal Outcomes in Fetal Growth Restriction and Small for Gestational Age.J Clin Med. 2022 May 12;11(10):2729. doi: 10.3390/jcm11102729. J Clin Med. 2022. PMID: 35628856 Free PMC article.
References
-
- Unterscheider J., O’Donoghue K., Daly S., Geary M.P., Kennelly M.M., McAuliffe F.M., Hunter A., Morrison J.J., Burke G., Dicker P., et al. Fetal growth restriction and the risk of perinatal mortality–Case studies from the multicentre PORTO study. BMC Pregnancy Childbirth. 2014;14:63. doi: 10.1186/1471-2393-14-63. - DOI - PMC - PubMed
-
- Marzouk A., Filipovic-Pierucci A., Baud O., Tsatsaris V., Ego A., Charles M.-A., Goffinet F., Evain-Brion D., Durand-Zaleski I. Prenatal and post-natal cost of small for gestational age infants: A national study. BMC Health Serv. Res. 2017;17:221. doi: 10.1186/s12913-017-2155-x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

