Effects of Calcium on Arsenate Adsorption and Arsenate/Iron Bioreduction of Ferrihydrite in Stimulated Groundwater
- PMID: 35329158
- PMCID: PMC8955117
- DOI: 10.3390/ijerph19063465
Effects of Calcium on Arsenate Adsorption and Arsenate/Iron Bioreduction of Ferrihydrite in Stimulated Groundwater
Abstract
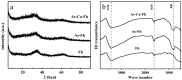
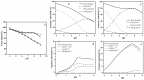
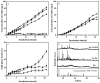
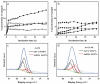
The reduction and transformation of arsenic-bearing ferrihydrite by arsenate-iron reducing bacteria is one of the main sources of arsenic enrichment in groundwater. During this process the coexistence cations may have a considerable effect. However, the ionic radius of calcium is larger than that of iron and shows a low affinity for ferrihydrite, and the effect of coexisting calcium on the migration and release of arsenic in arsenic-bearing ferrihydrite remains unclear. This study mainly explored the influence of adsorbed Ca2+ on strain JH012-1-mediated migration and release of arsenate in a simulated groundwater environment, in which 3 mM ferrihydrite and pH 7.5. Ca2+ were pre-absorbed on As(V)-containing ferrihydrite with a As:Fe ratio of 0.2. Solid samples were analyzed by X-ray diffraction (XRD), scanning electron microscopic (SEM), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). The results show that calcium and arsenate can synergistically adsorb on ferrihydrite due to the electrostatic interactions, and the adsorbed Ca2+ mainly exists on the surface through the outer-sphere complex. Adsorbed Ca2+ entering the stimulated groundwater was easily disturbed and led to an extra release of 3.5 mg/L arsenic in the early stage. Moreover, adsorbed Ca2+ inhibited biogenic ferrous ions from accumulating on ferrihydrite. As a result, only 12.30% Fe(II) existed in the solid phase, whereas 29.35% existed without Ca2+ adsorption. Thus, the generation of parasymplesite was inhibited, which is not conducive to the immobilization of arsenic in groundwater.
Keywords: As bioreduction; calcium; electrostatic adsorption; ferrihydrite; groundwater.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Influence of Zn(II) on the adsorption of arsenate onto ferrihydrite.Environ Sci Technol. 2012 Dec 18;46(24):13152-9. doi: 10.1021/es300729m. Epub 2012 Dec 3. Environ Sci Technol. 2012. PMID: 23170764
-
Coprecipitated arsenate inhibits thermal transformation of 2-line ferrihydrite: implications for long-term stability of ferrihydrite.Chemosphere. 2015 Mar;122:88-93. doi: 10.1016/j.chemosphere.2014.11.017. Epub 2014 Nov 26. Chemosphere. 2015. PMID: 25433978
-
Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)oxides.Environ Sci Technol. 2016 Mar 1;50(5):2281-91. doi: 10.1021/acs.est.5b04625. Epub 2016 Feb 18. Environ Sci Technol. 2016. PMID: 26828118
-
Enhancing the bioreduction and interaction of arsenic and iron by thiosulfate in groundwater.Ecotoxicol Environ Saf. 2024 Apr 1;274:116210. doi: 10.1016/j.ecoenv.2024.116210. Epub 2024 Mar 13. Ecotoxicol Environ Saf. 2024. PMID: 38479311
-
Application of infrared spectroscopy and its theoretical simulation to arsenic adsorption processes.Water Environ Res. 2023 Apr;95(4):e10867. doi: 10.1002/wer.10867. Water Environ Res. 2023. PMID: 37041692 Review.
Cited by
-
Polycaprolactone-Modified Biochar Supported Nanoscale Zero-Valent Iron Coupling with Shewanella putrefaciens CN32 for 1,1,1-Trichloroethane Removal from Simulated Groundwater: Synthesis, Optimization, and Mechanism.Molecules. 2023 Mar 31;28(7):3145. doi: 10.3390/molecules28073145. Molecules. 2023. PMID: 37049906 Free PMC article.
-
Removal of Arsenic(V) from wastewater using calcined eggshells as a cost-effective adsorbent.Heliyon. 2025 Feb 6;11(3):e42505. doi: 10.1016/j.heliyon.2025.e42505. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 40007776 Free PMC article.
References
-
- Palma-Lara I., Martínez-Castillo M., Quintana-Pérez J., Arellano-Mendoza M., Tamay-Cach F., Valenzuela-Limón O., García-Montalvo E., Hernández-Zavala A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020;110:104539. doi: 10.1016/j.yrtph.2019.104539. - DOI - PubMed
-
- Muehe E.M., Kappler A. Arsenic mobility and toxicity in South and South-east Asia–a review on biogeochemistry, health and socio-economic effects, remediation and risk predictions. Environ. Chem. 2014;11:483–495. doi: 10.1071/EN13230. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

