Awareness and Perceptions among Members of a Japanese Cancer Patient Advocacy Group Concerning the Financial Relationships between the Pharmaceutical Industry and Physicians
- PMID: 35329160
- PMCID: PMC8952770
- DOI: 10.3390/ijerph19063478
Awareness and Perceptions among Members of a Japanese Cancer Patient Advocacy Group Concerning the Financial Relationships between the Pharmaceutical Industry and Physicians
Abstract
Objectives: Awareness and perceptions of financial conflicts of interest (FCOI) between pharmaceutical companies (Pharma) and healthcare domains remain unclear in Japanese cancer patient communities. This study aimed to assess awareness (RQ1), the influence of FCOI on physician trustworthiness (RQ2), and their perception (RQ3) among the Japanese cancer patient advocacy group members.
Methods: A cross-sectional study using a self-administered survey was conducted with a Japanese cancer patient advocacy group between January and February 2019. The main outcome measures included awareness and perceptions of physician-Pharma interactions, their impact on physician trustworthiness, and attitudes towards FCOI among medical and other professions. Furthermore, we performed thematic analyses on the comments which responders provided in the surveys.
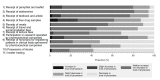
Results: Among the 524 contacted members, 96 (18.3%) completed the questionnaire, including 69 (77.5%) cancer patients. In RQ1, most of the respondents were aware of physician-Pharma interactions, although the extent differed based on the nature of the interaction. Furthermore, the respondents mainly considered these interactions influential on clinical practice (RQ2) and agreed to the need for further regulation of physician-Pharma interactions (QR3). In qualitative analyses (n = 56), we identified the 4 following themes: perception towards the FCOI (Theme 1), concerns about the respondent's treatment (Theme 2), reason of physician-Pharma interactions (Theme 3), and possible solutions from the patient perspective (Theme 4).
Conclusions: Most respondents were generally aware of physician-Pharma-associated FCOI and perceived them negatively. Additionally, participants appeared supportive of further FCOI regulation to protect patient-centred care.
Abbreviations: FCOI-financial conflicts of interest; United States-US; Pharma-pharmaceutical companies; RQ-research question.
Keywords: Japan; conflict of interest; ethics; financial relationship; patient-centred care; pharmaceutical industry.
Conflict of interest statement
Saito receives personal fees from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan), outside the scope of the submitted work. Ozaki receives personal fees from Medical Network Systems outside the scope of the submitted work. Tanimoto receives personal fees from Medical Network Systems (Tokyo, Japan) and Bionics Co. Ltd. (Tokyo, Japan), outside the scope of the submitted work. The remaining authors declare no financial conflicts of interest. Anju Murayama, Yuki Senoo, Kayo Harada, Hiroaki Saito, Toyoaki Sawano, Tetsuya Tanimoto, and Akihiko Ozaki report a number of studies on conflicts of interest in Japan.
Figures




References
-
- Institute of Medicine Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; Washington, DC, USA: 2001. - PubMed
-
- Ozaki A., Saito H., Senoo Y., Sawano T., Shimada Y., Kobashi Y., Yamamoto K., Suzuki Y., Tanimoto T. Overview and transparency of non-research payments to healthcare organizations and healthcare professionals from pharmaceutical companies in Japan: Analysis of payment data in 2016. Health Policy. 2020;124:727–735. doi: 10.1016/j.healthpol.2020.03.011. - DOI - PubMed
-
- Fickweiler F., Fickweiler W., Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: A systematic review. Br. Med. J. Open. 2017;7:e016408. doi: 10.1136/bmjopen-2017-016408. - DOI - PMC - PubMed
-
- Goupil B., Balusson F., Naudet F., Esvan M., Bastian B., Chapron A., Frouard P. Association between gifts from pharmaceutical companies to French general practitioners and their drug prescribing patterns in 2016: Retrospective study using the French Transparency in Healthcare and National Health Data System databases. Br. Med. J. 2019;367:l6015. doi: 10.1136/bmj.l6015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

