No Excess Mortality up to 10 Years in Early Stages of Breast Cancer in Women Adherent to Oral Endocrine Therapy: A Probabilistic Graphical Modeling Approach
- PMID: 35329292
- PMCID: PMC8950380
- DOI: 10.3390/ijerph19063605
No Excess Mortality up to 10 Years in Early Stages of Breast Cancer in Women Adherent to Oral Endocrine Therapy: A Probabilistic Graphical Modeling Approach
Abstract
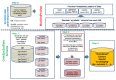
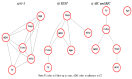
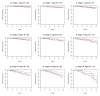
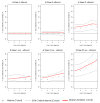
Breast cancer (BC) is globally the most frequent cancer in women. Adherence to endocrine therapy (ET) in hormone-receptor-positive BC patients is active and voluntary for the first five years after diagnosis. This study examines the impact of adherence to ET on 10-year excess mortality (EM) in patients diagnosed with Stages I to III BC (N = 2297). Since sample size is an issue for estimating age- and stage-specific survival indicators, we developed a method, ComSynSurData, for generating a large synthetic dataset (SynD) through probabilistic graphical modeling of the original cohort. We derived population-based survival indicators using a Bayesian relative survival model fitted to the SynD. Our modeling showed that hormone-receptor-positive BC patients diagnosed beyond 49 years of age at Stage I or beyond 59 years at Stage II do not have 10-year EM if they follow the prescribed ET regimen. This result calls for developing interventions to promote adherence to ET in patients with hormone receptor-positive BC and in turn improving cancer survival. The presented methodology here demonstrates the potential use of probabilistic graphical modeling for generating reliable synthetic datasets for validating population-based survival indicators when sample size is an issue.
Keywords: adherence; breast cancer; endocrine therapy; excess mortality; graphical modeling; synthetic dataset.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Using population-based data to evaluate the impact of adherence to endocrine therapy on survival in breast cancer through the web-application BreCanSurvPred.Sci Rep. 2022 May 16;12(1):8097. doi: 10.1038/s41598-022-12228-y. Sci Rep. 2022. PMID: 35577853 Free PMC article.
-
Survival Outcomes of Early-Stage Hormone Receptor-Positive Breast Cancer in Elderly Women.Ann Surg Oncol. 2020 Nov;27(12):4853-4860. doi: 10.1245/s10434-020-08945-1. Epub 2020 Sep 11. Ann Surg Oncol. 2020. PMID: 32918178
-
Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer.Cancer. 2019 Sep 15;125(18):3266-3274. doi: 10.1002/cncr.32192. Epub 2019 May 23. Cancer. 2019. PMID: 31120571
-
Endocrine adherence in male versus female breast cancer: a seer-medicare review.Breast Cancer Res Treat. 2022 Apr;192(3):491-499. doi: 10.1007/s10549-022-06536-0. Epub 2022 Feb 10. Breast Cancer Res Treat. 2022. PMID: 35142938 Review.
-
Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence?J Cancer Surviv. 2018 Jun;12(3):348-356. doi: 10.1007/s11764-017-0674-4. Epub 2018 Feb 2. J Cancer Surviv. 2018. PMID: 29396760 Review.
Cited by
-
Ten-Year Probabilities of Death Due to Cancer and Cardiovascular Disease among Breast Cancer Patients Diagnosed in North-Eastern Spain.Int J Environ Res Public Health. 2022 Dec 27;20(1):405. doi: 10.3390/ijerph20010405. Int J Environ Res Public Health. 2022. PMID: 36612726 Free PMC article.
References
-
- Chirlaque M.D., Salmerón D., Galceran J., Ameijide A., Mateos A., Torrella A., Jiménez R., Larrañaga N., Marcos-Gragera R., Ardanaz E., et al. Cancer survival in adult patients in Spain. Results from nine population-based cancer registries. Clin. Transl. Oncol. 2018;20:201–211. doi: 10.1007/s12094-017-1710-6. - DOI - PubMed
-
- Clèries R., Ameijide A., Buxó M., Martínez J.M., Marcos-Gragera R., Vilardell M.-L., Carulla M., Yasui Y., Vilardell M., Espinàs J.A., et al. Long-term crude probabilities of death among breast cancer patients by age and stage: A population-based survival study in Northeastern Spain (Girona–Tarragona 1985–2004) Clin. Transl. Oncol. 2018;20:1252–1260. doi: 10.1007/s12094-018-1852-1. - DOI - PMC - PubMed

