Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review
- PMID: 35329474
- PMCID: PMC8954067
- DOI: 10.3390/ma15062023
Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review
Abstract
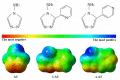
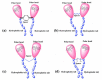
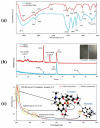
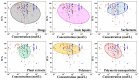
Most studies on the corrosion inhibition performance of organic molecules and (nano)materials were conducted within "carbon steel/1.0 M HCl" solution system using similar experimental and theoretical methods. As such, the numerous research findings in this system are sufficient to conduct comparative studies to select the best-suited inhibitor type that generally refers to a type of inhibitor with low concentration/high inhibition efficiency, nontoxic properties, and a simple and cost-economic synthesis process. Before data collection, to help readers have a clear understanding of some crucial elements for the evaluation of corrosion inhibition performance, we introduced the mainstay of corrosion inhibitors studies involved, including the corrosion and inhibition mechanism of carbon steel/HCl solution systems, evaluation methods of corrosion inhibition efficiency, adsorption isotherm models, adsorption thermodynamic parameters QC calculations, MD/MC simulations, and the main characterization techniques used. In the classification and statistical analysis section, organic compounds or (nano)materials as corrosion inhibitors were classified into six types according to their molecular structural characteristics, molecular size, and compound source, including drug molecules, ionic liquids, surfactants, plant extracts, polymers, and polymeric nanoparticles. We outlined the important conclusions obtained from recent literature and listed the evaluation methods, characterization techniques, and contrastable experimental data of these types of inhibitors when used for carbon steel corrosion in 1.0 M HCl solution. Finally, statistical analysis was only performed based on these data from carbon steel/1.0 M HCl solution system, from which some conclusions can contribute to reducing the workload of the acquisition of useful information and provide some reference directions for the development of new corrosion inhibitors.
Keywords: 1.0 M HCl; carbon steel; corrosion inhibition performance; evaluation method; statistical analysis.
Conflict of interest statement
The authors declare that there are no known competing financial interests or personal relationships in this paper.
Figures
















Similar articles
-
Polymeric organic Amberlite™ IRC-200 extract resin compound as a novel corrosion inhibitor for carbon steel in 1.0 M HCl acid: detail experimental, surface, molecular studies (DFT + MC/MD) and kinetic isotherm adsorption.Environ Sci Pollut Res Int. 2025 Feb;32(9):5551-5573. doi: 10.1007/s11356-025-35884-9. Epub 2025 Feb 11. Environ Sci Pollut Res Int. 2025. PMID: 39932609
-
Synthesis of polyaniline tannate-modified carbon nanotubes by oxidative copolymerization as highly efficient corrosion inhibitors for mild steel in HCl solution.J Colloid Interface Sci. 2025 Feb 15;680(Pt A):479-495. doi: 10.1016/j.jcis.2024.10.189. Epub 2024 Nov 1. J Colloid Interface Sci. 2025. PMID: 39522243
-
An investigation on the synthesis, characterization and anti-corrosion properties of choline based ionic liquids as novel and environmentally friendly inhibitors for mild steel corrosion in 5% HCl.J Colloid Interface Sci. 2022 Aug 15;620:293-312. doi: 10.1016/j.jcis.2022.04.036. Epub 2022 Apr 10. J Colloid Interface Sci. 2022. PMID: 35429708
-
A Brief Review on Fruit and Vegetable Extracts as Corrosion Inhibitors in Acidic Environments.Molecules. 2022 May 6;27(9):2991. doi: 10.3390/molecules27092991. Molecules. 2022. PMID: 35566341 Free PMC article. Review.
-
Advancements in ionic liquid-based corrosion inhibitors for sustainable protection strategies: from experimental to computational insights.Adv Colloid Interface Sci. 2024 Nov;333:103303. doi: 10.1016/j.cis.2024.103303. Epub 2024 Sep 17. Adv Colloid Interface Sci. 2024. PMID: 39303355 Review.
Cited by
-
Structure-Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition.Int J Mol Sci. 2025 Jun 16;26(12):5750. doi: 10.3390/ijms26125750. Int J Mol Sci. 2025. PMID: 40565214 Free PMC article.
-
4-amino-5-(trifluoromethyl)-4H-1, 2, 4-triazole-3-thiol as an effective corrosion inhibitor for low carbon steel in HCl environment: experimental and theoretical studies.BMC Chem. 2025 Jul 10;19(1):205. doi: 10.1186/s13065-025-01553-8. BMC Chem. 2025. PMID: 40640917 Free PMC article.
-
Novel porphyrin derivatives as corrosion inhibitors for stainless steel 304 in acidic environment: synthesis, electrochemical and quantum calculation studies.Sci Rep. 2023 Oct 16;13(1):17593. doi: 10.1038/s41598-023-44873-2. Sci Rep. 2023. PMID: 37845330 Free PMC article.
-
Electrochemical Assessment of Rhus typhina L. Leaf Extract as a Novel Green Corrosion Inhibitor for OL37 in 1 M HCl Medium.Molecules. 2025 Jun 19;30(12):2660. doi: 10.3390/molecules30122660. Molecules. 2025. PMID: 40572622 Free PMC article.
-
A pH-Controlled Solid Inhibitor Based on PAM Hydrogel for Steel Corrosion Protection in Wide Range pH NaCl Medium.Molecules. 2023 Jan 30;28(3):1314. doi: 10.3390/molecules28031314. Molecules. 2023. PMID: 36770984 Free PMC article.
References
-
- Choi Y.-S., Shim J.-J., Kim J.-G. Effects of Cr, Cu, Ni and Ca on the corrosion behavior of low carbon steel in synthetic tap water. J. Alloys Compd. 2005;391:162–169. doi: 10.1016/j.jallcom.2004.07.081. - DOI
-
- Li D., Feng Y., Bai Z., Zhu J., Zheng M. Influence of temperature, chloride ions and chromium element on the electronic property of passive film formed on carbon steel in bicarbonate/carbonate buffer solution. Electrochim. Acta. 2007;52:7877–7884. doi: 10.1016/j.electacta.2007.06.059. - DOI
-
- Liu C., Revilla R.I., Liu Z., Zhang D., Li X., Terryn H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017;129:82–90. doi: 10.1016/j.corsci.2017.10.001. - DOI
-
- Ma Q., Tong Z., Wang W., Dong G. Fabricating robust and repairable superhydrophobic surface on carbon steel by nanosecond laser texturing for corrosion protection. Appl. Surf. Sci. 2018;455:748–757. doi: 10.1016/j.apsusc.2018.06.033. - DOI
-
- Shibaeva T.V., Laurinavichyute V., Tsirlina G., Arsenkin A.M., Grigorovich K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014;80:299–308. doi: 10.1016/j.corsci.2013.11.038. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

