Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering-In Search of Relevant Excipients for Pharmaceutical 3D Printing
- PMID: 35329594
- PMCID: PMC8950795
- DOI: 10.3390/ma15062142
Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering-In Search of Relevant Excipients for Pharmaceutical 3D Printing
Abstract
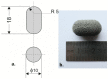
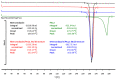
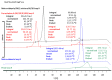
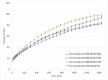
3D printing by selective laser sintering (SLS) of high-dose drug delivery systems using pure brittle crystalline active pharmaceutical ingredients (API) is possible but impractical. Currently used pharmaceutical grade excipients, including polymers, are primarily designed for powder compression, ensuring good mechanical properties. Using these excipients for SLS usually leads to poor mechanical properties of printed tablets (printlets). Composite printlets consisting of sintered carbon-stained polyamide (PA12) and metronidazole (Met) were manufactured by SLS to overcome the issue. The printlets were characterized using DSC and IR spectroscopy together with an assessment of mechanical properties. Functional properties of the printlets, i.e., drug release in USP3 and USP4 apparatus together with flotation assessment, were evaluated. The printlets contained 80 to 90% of Met (therapeutic dose ca. 600 mg), had hardness above 40 N (comparable with compressed tablets) and were of good quality with internal porous structure, which assured flotation. The thermal stability of the composite material and the identity of its constituents were confirmed. Elastic PA12 mesh maintained the shape and structure of the printlets during drug dissolution and flotation. Laser speed and the addition of an osmotic agent in low content influenced drug release virtually not changing composition of the printlet; time to release 80% of Met varied from 0.5 to 5 h. Composite printlets consisting of elastic insoluble PA12 mesh filled with high content of crystalline Met were manufactured by 3D SLS printing. Dissolution modification by the addition of an osmotic agent was demonstrated. The study shows the need to define the requirements for excipients dedicated to 3D printing and to search for appropriate materials for this purpose.
Keywords: 3D printing; composite materials; drug delivery; floating dosage forms; gastroretentive drug delivery systems; metronidazole; nylon; pharmaceutical additive manufacturing; polyamide 12; powder bed fusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Fabricating 3D printed orally disintegrating printlets using selective laser sintering.Int J Pharm. 2018 Apr 25;541(1-2):101-107. doi: 10.1016/j.ijpharm.2018.02.015. Epub 2018 Feb 14. Int J Pharm. 2018. PMID: 29454028
-
Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing.Int J Pharm. 2019 Oct 30;570:118651. doi: 10.1016/j.ijpharm.2019.118651. Epub 2019 Sep 4. Int J Pharm. 2019. PMID: 31493496
-
Very-Rapidly Dissolving Printlets of Isoniazid Manufactured by SLS 3D Printing: In Vitro and In Vivo Characterization.Mol Pharm. 2022 Aug 1;19(8):2937-2949. doi: 10.1021/acs.molpharmaceut.2c00306. Epub 2022 Jun 1. Mol Pharm. 2022. PMID: 35648147 Free PMC article.
-
3D printing: Principles and pharmaceutical applications of selective laser sintering.Int J Pharm. 2020 Aug 30;586:119594. doi: 10.1016/j.ijpharm.2020.119594. Epub 2020 Jul 2. Int J Pharm. 2020. PMID: 32622811 Review.
-
Advances in powder bed fusion 3D printing in drug delivery and healthcare.Adv Drug Deliv Rev. 2021 Jul;174:406-424. doi: 10.1016/j.addr.2021.04.025. Epub 2021 May 2. Adv Drug Deliv Rev. 2021. PMID: 33951489 Review.
Cited by
-
Rising role of 3D-printing in delivery of therapeutics for infectious disease.J Control Release. 2024 Feb;366:349-365. doi: 10.1016/j.jconrel.2023.12.051. Epub 2024 Jan 8. J Control Release. 2024. PMID: 38182058 Free PMC article. Review.
-
3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering.Pharmaceutics. 2023 Aug 29;15(9):2230. doi: 10.3390/pharmaceutics15092230. Pharmaceutics. 2023. PMID: 37765199 Free PMC article.
-
3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope.Pharmaceutics. 2023 May 10;15(5):1448. doi: 10.3390/pharmaceutics15051448. Pharmaceutics. 2023. PMID: 37242690 Free PMC article. Review.
-
The Upper Limb Orthosis in the Rehabilitation of Stroke Patients: The Role of 3D Printing.Bioengineering (Basel). 2023 Oct 27;10(11):1256. doi: 10.3390/bioengineering10111256. Bioengineering (Basel). 2023. PMID: 38002380 Free PMC article. Review.
-
Printabily of Pharmaceutical-Grade Polymers using Selective Laser Sintering with a CO2 Laser.AAPS PharmSciTech. 2025 May 22;26(5):144. doi: 10.1208/s12249-025-03141-4. AAPS PharmSciTech. 2025. PMID: 40404930
References
-
- de Souza M.P.C., de Camargo B.A.F., Spósito L., Fortunato G.C., Carvalho G.C., Marena G.D., Meneguin A.B., Bauab T.M., Chorilli M. Highlighting the use of micro and nanoparticles based-drug delivery systems for the treatment of Helicobacter pylori infections. Crit. Rev. Microbiol. 2021;47:1–26. doi: 10.1080/1040841X.2021.1895721. - DOI - PubMed
-
- T3DB Version 2.0. [(accessed on 12 December 2021)]. Available online: http://www.t3db.ca/toxins/T3D4703.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

