Biodegradable Mg-Zn-Ca-Based Metallic Glasses
- PMID: 35329624
- PMCID: PMC8955783
- DOI: 10.3390/ma15062172
Biodegradable Mg-Zn-Ca-Based Metallic Glasses
Abstract
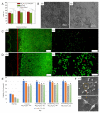
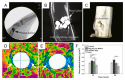
Biodegradable Mg-Zn-Ca-based metallic glasses (MGs) present improved strength and superior corrosion resistance, compared to crystalline Mg. In particular, in vivo and in vitro attempts reveal that biodegradable Mg-Zn-Ca-based MGs possess excellent biocompatibility, suggesting that they are ideal candidates for temporary implant materials. However, the limited size and severe brittleness prevent their widespread commercialization. In this review, we firstly summarize the microstructure characteristic and mechanical properties of Mg-Zn-Ca-based MGs. Then, we provide a comprehensive and systematic understanding of the recent progress of the biocorrosion and biocompatibility of Mg-Zn-Ca-based MGs. Last, but not least, the outlook towards the fabrication routes, composition design, structure design, and reinforcement approaches of Mg-Zn-Ca-based MGs are briefly proposed.
Keywords: Mg–Zn–Ca; biodegradable; implant; metallic glass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Composition-dependent structural and electronic properties of Mg(95-x)Zn(x)Ca5 metallic glasses: an ab initio molecular dynamics study.J Phys Chem B. 2015 Feb 26;119(8):3608-18. doi: 10.1021/acs.jpcb.5b00400. Epub 2015 Feb 16. J Phys Chem B. 2015. PMID: 25646858
-
Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites-A review.Acta Biomater. 2020 Feb;103:1-23. doi: 10.1016/j.actbio.2019.12.023. Epub 2019 Dec 24. Acta Biomater. 2020. PMID: 31881312 Review.
-
A Critical Review on Metallic Glasses as Structural Materials for Cardiovascular Stent Applications.J Funct Biomater. 2018 Feb 27;9(1):19. doi: 10.3390/jfb9010019. J Funct Biomater. 2018. PMID: 29495521 Free PMC article. Review.
-
Recent advances in bulk metallic glasses for biomedical applications.Acta Biomater. 2016 May;36:1-20. doi: 10.1016/j.actbio.2016.03.047. Epub 2016 Apr 1. Acta Biomater. 2016. PMID: 27045349 Review.
-
Microstructure, mechanical properties, biocompatibility, and in vitro corrosion and degradation behavior of a new Zn-5Ge alloy for biodegradable implant materials.Acta Biomater. 2018 Dec;82:197-204. doi: 10.1016/j.actbio.2018.10.015. Epub 2018 Oct 11. Acta Biomater. 2018. PMID: 30316837
Cited by
-
Biocompatibility of a Zr-Based Metallic Glass Enabled by Additive Manufacturing.ACS Appl Bio Mater. 2022 Dec 19;5(12):5741-5753. doi: 10.1021/acsabm.2c00764. Epub 2022 Dec 2. ACS Appl Bio Mater. 2022. PMID: 36459395 Free PMC article.
-
A Review of the Development of Titanium-Based and Magnesium-Based Metallic Glasses in the Field of Biomedical Materials.Materials (Basel). 2024 Sep 19;17(18):4587. doi: 10.3390/ma17184587. Materials (Basel). 2024. PMID: 39336328 Free PMC article. Review.
-
Long-Term Examination of Degradation and In Vivo Biocompatibility of Some Mg-0.5Ca-xY Alloys in Sprague Dawley Rats.Materials (Basel). 2022 Aug 29;15(17):5958. doi: 10.3390/ma15175958. Materials (Basel). 2022. PMID: 36079340 Free PMC article.
-
Evaluation of Physiochemical and Biological Properties of Biofunctionalized Mg-Based Implants Obtained via Large-Scale PEO Process for Dentistry Applications.J Funct Biomater. 2023 Jun 27;14(7):338. doi: 10.3390/jfb14070338. J Funct Biomater. 2023. PMID: 37504833 Free PMC article.
-
Improving the Mechanical Properties of Mg-5Al-2Ca-1Mn-0.5Zn Alloy through Rotary Swaging.Materials (Basel). 2023 Jun 20;16(12):4489. doi: 10.3390/ma16124489. Materials (Basel). 2023. PMID: 37374672 Free PMC article.
References
-
- Yang Y., He C., Dianyu E., Yang W., Qi F., Xie D., Shen L., Peng S., Shuai C. Mg bone implant: Features, developments and perspectives. Mater. Design. 2020;185:108259. doi: 10.1016/j.matdes.2019.108259. - DOI
-
- Li K.H., Ge J.C., Liu S.N., Fu S., Yin Z.X., Zhang W.T., Chen G.X., Wei S.C., Ji H., Feng T., et al. In situ scattering study of multiscale structural evolution during liquid–liquid phase transition in Mg-based metallic glasses. Rare Met. 2021;40:3107–3116. doi: 10.1007/s12598-021-01767-4. - DOI
-
- Sezer N., Evis Z., Kayhan S.M., Tahmasebifar A., Koç M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018;6:23–43. doi: 10.1016/j.jma.2018.02.003. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

