Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area
- PMID: 35329627
- PMCID: PMC8950381
- DOI: 10.3390/ma15062176
Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area
Abstract
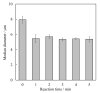
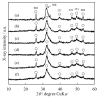
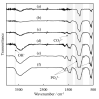
Chelate-setting calcium-phosphate cements (CPCs) have been developed using inositol phosphate (IP6) as a chelating agent. However, the compressive strength of the CPC fabricated from a commercially available hydroxyapatite (HAp) powder was approximately 10 MPa. In this study, we miniaturized HAp particles as a starting powder to improve the compressive strength of chelate-setting CPCs and examined the adsorption properties of IP6 onto HAp powders. An HAp powder with a specific surface area (SSA) higher than 200 m2/g (HApHS) was obtained by ultrasonic irradiation for 1 min in a wet synthesis process, greatly improving the SSA (214 m2/g) of the commercial powder without ultrasonic irradiation. The HApHS powder was found to be a B-type carbonate-containing HAp in which the phosphate groups in apatite were replaced by carbonate groups. Owing to the high SSA, the HApHS powder also showed high IP6 adsorption capacity. The adsorption phenomena of IP6 to our HApHS and commercially available Hap powders were found to follow the Freundlich and Langmuir models, respectively. We found that IP6 adsorbs on the HApHS powder by both physisorption and chemisorption. The fine HapHS powder with a high SSA is a novel raw powder material, expected to improve the compressive strength of chelate-setting CPCs.
Keywords: Hydroxyapatite; adsorption; chelate-setting cement; inositol phosphate; ultrasonic irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Novel chelate-setting calcium-phosphate cements fabricated with wet-synthesized hydroxyapatite powder.J Mater Sci Mater Med. 2013 Mar;24(3):611-21. doi: 10.1007/s10856-012-4834-9. Epub 2012 Dec 11. J Mater Sci Mater Med. 2013. PMID: 23229575
-
Injectable chelate-setting hydroxyapatite cement prepared by using chitosan solution: Fabrication, material properties, biocompatibility, and osteoconductivity.J Biomater Appl. 2017 May;31(10):1319-1327. doi: 10.1177/0885328217704060. J Biomater Appl. 2017. PMID: 28517977
-
Bioresorbable porous β-tricalcium phosphate chelate-setting cements with poly(lactic-co-glycolic acid) particles as pore-forming agent: fabrication, material properties, cytotoxicity, and in vivo evaluation.Sci Technol Adv Mater. 2021 Jun 24;22(1):511-521. doi: 10.1080/14686996.2021.1936628. Sci Technol Adv Mater. 2021. PMID: 34220339 Free PMC article.
-
Effects of Adding Polysaccharides and Citric Acid into Sodium Dihydrogen Phosphate Mixing Solution on the Material Properties of Gelatin-Hybridized Calcium-Phosphate Cement.Materials (Basel). 2017 Aug 12;10(8):941. doi: 10.3390/ma10080941. Materials (Basel). 2017. PMID: 28805704 Free PMC article.
-
Transforming growth factor-beta1 incorporation in a calcium phosphate bone cement: material properties and release characteristics.J Biomed Mater Res. 2002 Feb;59(2):265-72. doi: 10.1002/jbm.1241. J Biomed Mater Res. 2002. PMID: 11745562
Cited by
-
Preparation and Characterization of Magnetite Talc (Fe3O4@Talc) Nanocomposite as an Effective Adsorbent for Cr(VI) and Alizarin Red S Dye.Materials (Basel). 2022 May 9;15(9):3401. doi: 10.3390/ma15093401. Materials (Basel). 2022. PMID: 35591732 Free PMC article.
-
Phytic Acid Demonstrates Rapid Antibiofilm Activity and Inhibits Biofilm Formation When Used as a Surface Conditioning Agent.Microbiol Spectr. 2023 Jun 15;11(3):e0026723. doi: 10.1128/spectrum.00267-23. Epub 2023 May 16. Microbiol Spectr. 2023. PMID: 37191582 Free PMC article.
-
Phosphorus Interactions with Iron in Undisturbed and Disturbed Arctic Tundra Ecosystems.Environ Sci Technol. 2024 Jul 2;58(26):11400-11410. doi: 10.1021/acs.est.3c09072. Epub 2024 Jun 18. Environ Sci Technol. 2024. PMID: 38889135 Free PMC article.
References
-
- Wen S., Liu X., Ding J., Liu Y., Lan Z., Zhang Z., Chen G. Hydrothermal Synthesis of Hydroxyapatite Coating on the Surface of Medical Magnesium Alloy and Its Corrosion Resistance. Prog. Nat. Sci. Mater. Int. 2021;31:324–333. doi: 10.1016/j.pnsc.2020.12.013. - DOI
-
- Le Gars Santoni B., Niggli L., Dolder S., Loeffel O., Sblendorio G.A., Heuberger R., Maazouz Y., Stähli C., Döbelin N., Bowen P., et al. Effect of Minor Amounts of β-Calcium Pyrophosphate and Hydroxyapatite on the Physico-Chemical Properties and Osteoclastic Resorption of β-Tricalcium Phosphate Cylinders. Bioact. Mater. 2022;10:222–235. doi: 10.1016/j.bioactmat.2021.09.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

