Removal of Thiol-SAM on a Gold Surface for Re-Use of an Interdigitated Chain-Shaped Electrode
- PMID: 35329670
- PMCID: PMC8950519
- DOI: 10.3390/ma15062218
Removal of Thiol-SAM on a Gold Surface for Re-Use of an Interdigitated Chain-Shaped Electrode
Abstract
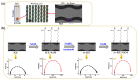
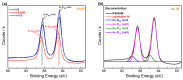
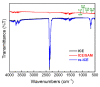
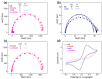
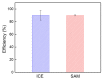
The self-assembled monolayer (SAM) is the most common organic assembly utilized for the formation of the monolayers of alkane-thiolates on gold electrode, resulting in a wide range of applications for the modified SAM on gold in various research areas. This study examined the desorption of a SAM that was developed on the gold surface of an interdigitated chain-shaped electrode (the ICE, a unique electrode design, was fabricated by our group) with the goal of determining the most efficient strategy of SAM removal for the ICE to be re-used. A simple and proficient solution-based cleaning procedure was applied for the removal of a SAM on the gold surface of the ICE by using a sodium borohydride solution within short-term treatment, resulting in efficiency for the recovery of the originally electrochemical characteristic of ICE of 90.3%. The re-use of ICE after the removal process was confirmed by the successful re-deposition of a SAM onto the electrode surface, resulting in the high efficiency percentage of 90.1% for the reusability of ICE with the SAM modification. Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were used as tools to investigate the changes in the electrode interface at each stage of the SAM removal and the electrode recycling. X-ray photoelectron spectroscopy and Fourier-transform infrared spectroscopy were employed, being powerful spectrum techniques, for the characterization of the bonding structure and chemical state of the bare ICE and the modified ICE at each treatment step. Based on the comprehensive discussion of analytical chemistry from the obtained EIS and CV data in this study, we confirmed and proved the effectiveness of this promising method for the removal of a SAM from the ICE and the re-use of ICE in the field of material deposition, with the aims of saving money, improving experimental handling, and protecting the environment.
Keywords: SAM removal; electrochemical impedance spectroscopy; interdigitated chain-shaped electrode; self-assembled monolayer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Electrochemical Detection of C-Reactive Protein in Human Serum Based on Self-Assembled Monolayer-Modified Interdigitated Wave-Shaped Electrode.Sensors (Basel). 2019 Dec 16;19(24):5560. doi: 10.3390/s19245560. Sensors (Basel). 2019. PMID: 31888286 Free PMC article.
-
Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode.Biosens Bioelectron. 2019 Nov 1;144:111694. doi: 10.1016/j.bios.2019.111694. Epub 2019 Sep 7. Biosens Bioelectron. 2019. PMID: 31539720
-
Electrochemical and X-ray Photoelectron Spectroscopy Surface Characterization of Interchain-Driven Self-Assembled Monolayer (SAM) Reorganization.Nanomaterials (Basel). 2022 Mar 4;12(5):867. doi: 10.3390/nano12050867. Nanomaterials (Basel). 2022. PMID: 35269355 Free PMC article.
-
Electrocrystallization of rhodium clusters on thiolate-covered polycrystalline gold.Langmuir. 2005 May 24;21(11):5124-33. doi: 10.1021/la050078z. Langmuir. 2005. PMID: 15896060
-
Surface Protection of Quaternary Gold Alloys by Thiol Self-Assembled Monolayers.Int J Mol Sci. 2022 Nov 16;23(22):14132. doi: 10.3390/ijms232214132. Int J Mol Sci. 2022. PMID: 36430610 Free PMC article.
Cited by
-
SAM-Support-Based Electrochemical Sensor for Aβ Biomarker Detection of Alzheimer's Disease.Biosensors (Basel). 2023 Aug 11;13(8):809. doi: 10.3390/bios13080809. Biosensors (Basel). 2023. PMID: 37622895 Free PMC article. Review.
-
Using Nanomaterials for SARS-CoV-2 Sensing via Electrochemical Techniques.Micromachines (Basel). 2023 Apr 25;14(5):933. doi: 10.3390/mi14050933. Micromachines (Basel). 2023. PMID: 37241556 Free PMC article. Review.
-
Novel High-Throughput Electrochemical Detection of Staphylococcus Aureus, Bacillus Cereus, or Micrococcus Luteus Using AuNPs@Ti3C2Tz Functionalized with Sandwich Peptides.Small. 2025 Jul;21(29):e2411486. doi: 10.1002/smll.202411486. Epub 2025 Mar 18. Small. 2025. PMID: 40099964 Free PMC article.
References
-
- Hassanien A.S., Akl A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016;89:153–169. doi: 10.1016/j.spmi.2015.10.044. - DOI
-
- Hannachi A., Segura A., Meherzi H.M. Growth of manganese sulfide (α-MnS) thin films by thermal vacuum evaporation: Structural, morphological, optical properties. Mater. Chem. Phys. 2016;181:326–332. doi: 10.1016/j.matchemphys.2016.06.066. - DOI
-
- Lanzutti A., Lekka M., Leitenburg C., Fedrizzi L. Effect of pulse current on wear behavior of Ni matrix micro-and nano-SiC composite coatings at room and elevated temperature. Tribol. Int. 2019;132:50–61. doi: 10.1016/j.triboint.2018.12.011. - DOI
-
- Shourgeshty M., Aliofkhazraei M., Karimzadeh A. Study on functionally graded Zn–Ni–Al2O3 coatings fabricated by pulse-electrodeposition. Surf. Eng. 2019;35:167–176. doi: 10.1080/02670844.2018.1432172. - DOI
Grants and funding
- NRF2018M3A9F1023691/National Research Foundation of Korea
- NRF2019R1G1A1100610/National Research Foundation of Korea
- the "Technology Development Project for Biological Hazards Management in Indoor Air" Project, funded by Korea Ministry of Environment (MOE)(G232021010381)/Korea Environmental Industry and Technology Institute
- GRRC-Gachon2020(B01), AI-based Medical Image Analysis/GRRC program of Gyeonggi province
LinkOut - more resources
Full Text Sources

