Devising Bone Molecular Models at the Nanoscale: From Usual Mineralized Collagen Fibrils to the First Bone Fibers Including Hydroxyapatite in the Extra-Fibrillar Volume
- PMID: 35329726
- PMCID: PMC8955169
- DOI: 10.3390/ma15062274
Devising Bone Molecular Models at the Nanoscale: From Usual Mineralized Collagen Fibrils to the First Bone Fibers Including Hydroxyapatite in the Extra-Fibrillar Volume
Abstract
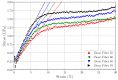
At the molecular scale, bone is mainly constituted of type-I collagen, hydroxyapatite, and water. Different fractions of these constituents compose different composite materials that exhibit different mechanical properties at the nanoscale, where the bone is characterized as a fiber, i.e., a bundle of mineralized collagen fibrils surrounded by water and hydroxyapatite in the extra-fibrillar volume. The literature presents only models that resemble mineralized collagen fibrils, including hydroxyapatite in the intra-fibrillar volume only, and lacks a detailed prescription on how to devise such models. Here, we present all-atom bone molecular models at the nanoscale, which, differently from previous bone models, include hydroxyapatite both in the intra-fibrillar volume and in the extra-fibrillar volume, resembling fibers in bones. Our main goal is to provide a detailed prescription on how to devise such models with different fractions of the constituents, and for that reason, we have made step-by-step scripts and files for reproducing these models available. To validate the models, we assessed their elastic properties by performing molecular dynamics simulations that resemble tensile tests, and compared the computed values against the literature (both experimental and computational results). Our results corroborate previous findings, as Young's Modulus values increase with higher fractions of hydroxyapatite, revealing all-atom bone models that include hydroxyapatite in both the intra-fibrillar volume and in the extra-fibrillar volume as a path towards realistic bone modeling at the nanoscale.
Keywords: bone elastic properties; bone nanoscale model; collagen fiber; extra-fibrillar volume; hydroxyapatite; mineralized collagen fibril; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Mohammad M.-G., Wang X.Z. Nanomechanics and Ultrastructure of Bone: A Review. Comput. Model. Eng. Sci. 2020;125:1–32. doi: 10.32604/cmes.2020.012123. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

