Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles
- PMID: 35329759
- PMCID: PMC8953906
- DOI: 10.3390/ma15062308
Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles
Abstract
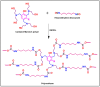
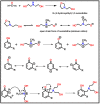
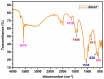
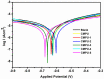
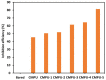
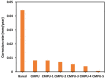
The present investigation demonstrates renewable cardanol-based polyol for the formulation of nanocomposite polyurethane (PU) coatings. The functional and structural features of cardanol polyol and nanoparticles were studied using FT-IR and 1H NMR spectroscopic techniques. The magnetic hydroxyapatite nanoparticles (MHAPs) were dispersed 1-5% in PU formulations to develop nanocomposite anticorrosive coatings. An increase in the strength of MHAP increased the anticorrosive performance as examined by immersion and electrochemical methods. The nanocomposite PU coatings showed good coating properties, viz., gloss, pencil hardness, flexibility, cross-cut adhesion, and chemical resistance. Additionally, the coatings were also studied for surface morphology, wetting, and thermal properties by scanning electron microscope (SEM), contact angle, and thermogravimetric analysis (TGA), respectively. The hydrophobic nature of PU coatings increased by the addition of MHAP, and an optimum result (105°) was observed in 3% loading. The developed coatings revealed its hydrophobic nature with excellent anticorrosive performance.
Keywords: anticorrosive coatings; cardanol; hydroxyapatite; nanocomposite; polyurethane; renewable materials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Vanadium Pentoxide-Enwrapped Polydiphenylamine/Polyurethane Nanocomposite: High-Performance Anticorrosive Coating.ACS Appl Mater Interfaces. 2019 Jan 16;11(2):2374-2385. doi: 10.1021/acsami.8b17861. Epub 2018 Dec 31. ACS Appl Mater Interfaces. 2019. PMID: 30561187
-
Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil.Polymers (Basel). 2023 Nov 12;15(22):4390. doi: 10.3390/polym15224390. Polymers (Basel). 2023. PMID: 38006114 Free PMC article.
-
Development of Metallo (Calcium/Magnesium) Polyurethane Nanocomposites for Anti-Corrosive Applications.Materials (Basel). 2022 Nov 24;15(23):8374. doi: 10.3390/ma15238374. Materials (Basel). 2022. PMID: 36499868 Free PMC article.
-
Nonedible Vegetable Oil-Based Polyols in Anticorrosive and Antimicrobial Polyurethane Coatings.Polymers (Basel). 2021 Sep 17;13(18):3149. doi: 10.3390/polym13183149. Polymers (Basel). 2021. PMID: 34578051 Free PMC article. Review.
-
Developments in anticorrosive organic coatings modulated by nano/microcontainers with porous matrices.Adv Colloid Interface Sci. 2024 Aug;330:103209. doi: 10.1016/j.cis.2024.103209. Epub 2024 Jun 4. Adv Colloid Interface Sci. 2024. PMID: 38848645 Review.
Cited by
-
Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products.Polymers (Basel). 2022 Dec 13;14(24):5456. doi: 10.3390/polym14245456. Polymers (Basel). 2022. PMID: 36559824 Free PMC article.
-
Recent Trends in Magnetic Polymer Nanocomposites for Aerospace Applications: A Review.Polymers (Basel). 2022 Sep 29;14(19):4084. doi: 10.3390/polym14194084. Polymers (Basel). 2022. PMID: 36236032 Free PMC article. Review.
-
Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide.Polymers (Basel). 2023 Feb 10;15(4):882. doi: 10.3390/polym15040882. Polymers (Basel). 2023. PMID: 36850165 Free PMC article.
-
Sustainable smart anti-corrosion coating materials derived from vegetable oil derivatives: a review.RSC Adv. 2023 Jan 27;13(6):3910-3941. doi: 10.1039/d2ra07825b. eCollection 2023 Jan 24. RSC Adv. 2023. PMID: 36756545 Free PMC article. Review.
-
The Effects of the Addition of Polyurethane-MgO Nanohybrids on the Mechanical Properties of Ordinary Portland Cement Paste.Nanomaterials (Basel). 2022 Nov 11;12(22):3978. doi: 10.3390/nano12223978. Nanomaterials (Basel). 2022. PMID: 36432264 Free PMC article.
References
-
- Bastidas D.M. Corrosion and protection of metals. Metals. 2020;10:458. doi: 10.3390/met10040458. - DOI
-
- Melchers R.E. A review of trends for corrosion loss and pit depth in longer-term exposures. Corros. Mater. Degrad. 2020;1:42–58. doi: 10.3390/cmd1010004. - DOI
-
- de la Fuente D., Díaz I., Simancas J., Chico B., Morcillo M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011;53:604–617. doi: 10.1016/j.corsci.2010.10.007. - DOI
-
- Paraskar P.M., Prabhudesai M.S., Hatkar V.M., Kulkarni R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021;156:106267. doi: 10.1016/j.porgcoat.2021.106267. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

