Experimental and Theoretical Investigations of Three-Ring Ester/Azomethine Materials
- PMID: 35329764
- PMCID: PMC8949326
- DOI: 10.3390/ma15062312
Experimental and Theoretical Investigations of Three-Ring Ester/Azomethine Materials
Abstract
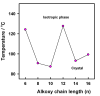
New three-ring ester/azomethine homologues series, (E)-4-((4-hydroxybenzylidene)amino)phenyl 4-(alkoxy)benzoate In, were prepared and their properties were investigated experimentally and theoretically. FT-IR, NMR, and elemental analyses were used to confirm the chemical structures of the synthesized compounds. The mesomorphic activities of the planned homologues were evaluated using differential scanning calorimetry (DSC) and polarized optical microscopy. All of the homologous examined were found to have non-mesomorphic properties. Theoretical calculations using the density functional theory (DFT) were used to validate the experimental data and determine the most stable conformation of the synthesized compounds. All calculated conformers' thermal properties, dipole moments, and polarizability were discussed. The results show that the terminal alkoxy chain length affects the thermal parameters of the conformers. The correlations between these parameters' values and the conformer type were demonstrated. The base component was expected to be in two conformers according to the orientation of the N atom of imine-linkage. DFT calculations revealed the more probable of the two possible conformers, and the incorporation of the alkoxy terminal chain in one position affect its geometrical and mesomerphic characteristics.
Keywords: DFT; conformational analysis; imine derivatives; mesomorphic properties; thermal parameters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
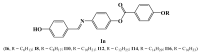




Similar articles
-
Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies.Molecules. 2022 Jun 28;27(13):4150. doi: 10.3390/molecules27134150. Molecules. 2022. PMID: 35807398 Free PMC article.
-
Synthetic, Mesomorphic, and DFT Investigations of New Nematogenic Polar Naphthyl Benzoate Ester Derivatives.Materials (Basel). 2021 May 16;14(10):2587. doi: 10.3390/ma14102587. Materials (Basel). 2021. PMID: 34065725 Free PMC article.
-
Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State.Molecules. 2021 Apr 2;26(7):2038. doi: 10.3390/molecules26072038. Molecules. 2021. PMID: 33918482 Free PMC article.
-
New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer.Polymers (Basel). 2022 Mar 21;14(6):1256. doi: 10.3390/polym14061256. Polymers (Basel). 2022. PMID: 35335586 Free PMC article.
-
Anisotropy (optical, electrical and thermal conductivity) in thin polarizing films for UV/Vis regions of spectrum: Experimental and theoretical investigations.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Mar 5;192:343-360. doi: 10.1016/j.saa.2017.11.029. Epub 2017 Nov 13. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29179085 Review.
Cited by
-
Design of Liquid Crystal Materials Based on Palmitate, Oleate, and Linoleate Derivatives for Optoelectronic Applications.Molecules. 2023 Feb 11;28(4):1744. doi: 10.3390/molecules28041744. Molecules. 2023. PMID: 36838732 Free PMC article.
-
Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies.Molecules. 2022 Jun 28;27(13):4150. doi: 10.3390/molecules27134150. Molecules. 2022. PMID: 35807398 Free PMC article.
-
Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations.Molecules. 2023 Mar 28;28(7):3019. doi: 10.3390/molecules28073019. Molecules. 2023. PMID: 37049783 Free PMC article.
-
Novel Imidazole Liquid Crystals; Experimental and Computational Approaches.Molecules. 2022 Jul 19;27(14):4607. doi: 10.3390/molecules27144607. Molecules. 2022. PMID: 35889474 Free PMC article.
References
-
- Arjmand F., Sayeed F., Muddassir M. Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. J. Photochem. Photobiol. B. Biol. 2011;103:166–179. doi: 10.1016/j.jphotobiol.2011.03.001. - DOI - PubMed
-
- Sayed A.R., Gomha S.M., El-Lateef H.M.A., Abolibda T.Z. L-proline catalyzed green synthesis and anticancer evaluation of novel bioactive benzil bis-hydrazones under grinding technique. Green Chem. Lett. Rev. 2021;14:180–189. doi: 10.1080/17518253.2021.1893392. - DOI
-
- Chandramouli C., Shivanand M.R., Nayanbhai T.B., Bheemachari B., Udupi R.H. Synthesis and biological screening of certain new triazole schiff bases and their derivatives bearing substituted benzothiazole moiety. J. Chem. Pharm. Res. 2012;4:1151–1159.
LinkOut - more resources
Full Text Sources

