Activation of the Carboxypeptidase U (CPU, TAFIa, CPB2) System in Patients with SARS-CoV-2 Infection Could Contribute to COVID-19 Hypofibrinolytic State and Disease Severity Prognosis
- PMID: 35329820
- PMCID: PMC8954233
- DOI: 10.3390/jcm11061494
Activation of the Carboxypeptidase U (CPU, TAFIa, CPB2) System in Patients with SARS-CoV-2 Infection Could Contribute to COVID-19 Hypofibrinolytic State and Disease Severity Prognosis
Abstract
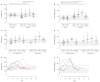
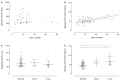
Coronavirus disease 2019 (COVID-19) is a viral lower respiratory tract infection caused by the highly transmissible and pathogenic SARS-CoV-2 (severe acute respiratory-syndrome coronavirus-2). Besides respiratory failure, systemic thromboembolic complications are frequent in COVID-19 patients and suggested to be the result of a dysregulation of the hemostatic balance. Although several markers of coagulation and fibrinolysis have been studied extensively, little is known about the effect of SARS-CoV-2 infection on the potent antifibrinolytic enzyme carboxypeptidase U (CPU). Blood was collected longitudinally from 56 hospitalized COVID-19 patients and 32 healthy controls. Procarboxypeptidase U (proCPU) levels and total active and inactivated CPU (CPU+CPUi) antigen levels were measured. At study inclusion (shortly after hospital admission), proCPU levels were significantly lower and CPU+CPUi antigen levels significantly higher in COVID-19 patients compared to controls. Both proCPU and CPU+CPUi antigen levels showed a subsequent progressive increase in these patients. Hereafter, proCPU levels decreased and patients were, at discharge, comparable to the controls. CPU+CPUi antigen levels at discharge were still higher compared to controls. Baseline CPU+CPUi antigen levels (shortly after hospital admission) correlated with disease severity and the duration of hospitalization. In conclusion, CPU generation with concomitant proCPU consumption during early SARS-CoV-2 infection will (at least partly) contribute to the hypofibrinolytic state observed in COVID-19 patients, thus enlarging their risk for thrombosis. Moreover, given the association between CPU+CPUi antigen levels and both disease severity and duration of hospitalization, this parameter may be a potential biomarker with prognostic value in SARS-CoV-2 infection.
Keywords: COVID-19; carboxypeptidase B2; carboxypeptidase U; coronavirus; thrombin-activatable fibrinolysis inhibitor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Carboxypeptidase U (CPU, TAFIa, CPB2) in Thromboembolic Disease: What Do We Know Three Decades after Its Discovery?Int J Mol Sci. 2021 Jan 17;22(2):883. doi: 10.3390/ijms22020883. Int J Mol Sci. 2021. PMID: 33477318 Free PMC article. Review.
-
Plasma carboxypeptidase U (CPU, CPB2, TAFIa) generation during in vitro clot lysis and its interplay between coagulation and fibrinolysis.Thromb Haemost. 2017 Jul 26;117(8):1498-1508. doi: 10.1160/TH17-02-0097. Epub 2017 Jul 6. Thromb Haemost. 2017. PMID: 28692110
-
Carboxypeptidase U (TAFIa) Is Rapidly Activated and Deactivated Following Thrombolysis and Thrombectomy in Stroke Patients.Transl Stroke Res. 2022 Dec;13(6):959-969. doi: 10.1007/s12975-021-00962-w. Epub 2021 Nov 19. Transl Stroke Res. 2022. PMID: 34796454
-
Plasma levels of carboxypeptidase U (CPU, CPB2 or TAFIa) are elevated in patients with acute myocardial infarction.J Thromb Haemost. 2015 Dec;13(12):2227-32. doi: 10.1111/jth.13135. Epub 2015 Nov 12. J Thromb Haemost. 2015. PMID: 26340515
-
An update on the role of carboxypeptidase U (TAFIa) in fibrinolysis.Front Biosci (Landmark Ed). 2011 Jun 1;16(7):2427-50. doi: 10.2741/3864. Front Biosci (Landmark Ed). 2011. PMID: 21622187 Review.
Cited by
-
SARS-CoV-2 infection results in upregulation of Plasminogen Activator Inhibitor-1 and Neuroserpin in the lungs, and an increase in fibrinolysis inhibitors associated with disease severity.EJHaem. 2023 Feb 23;4(2):324-338. doi: 10.1002/jha2.654. eCollection 2023 May. EJHaem. 2023. PMID: 37206290 Free PMC article.
-
Simple and Scalable Algorithms for Cluster-Aware Precision Medicine.Proc Mach Learn Res. 2024 May;238:136-144. Proc Mach Learn Res. 2024. PMID: 39015742 Free PMC article.
-
The suboptimal fibrinolytic response in COVID-19 is dictated by high PAI-1.J Thromb Haemost. 2022 Oct;20(10):2394-2406. doi: 10.1111/jth.15806. Epub 2022 Jul 21. J Thromb Haemost. 2022. PMID: 35780481 Free PMC article.
-
Treatment with intravenous immunoglobulin modulates coagulation- and complement-related pathways in COVID-19 patients.Front Immunol. 2025 Jul 31;16:1623309. doi: 10.3389/fimmu.2025.1623309. eCollection 2025. Front Immunol. 2025. PMID: 40821834 Free PMC article.
References
-
- World Health Organization (WHO) Coronavirus (COVID-19) [(accessed on 27 December 2021)]. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1.
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

