Enzyme-Linked Immunosorbent Assay: An Adaptable Methodology to Study SARS-CoV-2 Humoral and Cellular Immune Responses
- PMID: 35329828
- PMCID: PMC8948777
- DOI: 10.3390/jcm11061503
Enzyme-Linked Immunosorbent Assay: An Adaptable Methodology to Study SARS-CoV-2 Humoral and Cellular Immune Responses
Abstract
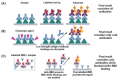
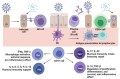
The Enzyme-Linked Immunosorbent Assay is a versatile technique, which can be used for several applications. It has enormously contributed to the study of infectious diseases. This review highlights how this methodology supported the science conducted in COVID-19 pandemics, allowing scientists to better understand the immune response against SARS-CoV-2. ELISA can be modified to assess the functionality of antibodies, as avidity and neutralization, respectively by the standardization of avidity-ELISA and surrogate-neutralization methods. Cellular immunity can also be studied using this assay. Products secreted by cells, like proteins and cytokines, can be studied by ELISA or its derivative Enzyme-linked immunospot (ELISpot) assay. ELISA and ELISA-based methods aided the area of immunology against infectious diseases and is still relevant, for example, as a promising approach to study the differences between natural and vaccine-induced immune responses against SARS-CoV-2.
Keywords: ELISA; SARS-CoV-2; antibody; cytokine; immune response.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this article.
Figures

Similar articles
-
Evaluating Humoral Immunity against SARS-CoV-2: Validation of a Plaque-Reduction Neutralization Test and a Multilaboratory Comparison of Conventional and Surrogate Neutralization Assays.Microbiol Spectr. 2021 Dec 22;9(3):e0088621. doi: 10.1128/Spectrum.00886-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787495 Free PMC article.
-
Dynamics of humoral and cellular immune responses after homologous and heterologous SARS-CoV-2 vaccination with ChAdOx1 nCoV-19 and BNT162b2.EBioMedicine. 2022 Nov;85:104294. doi: 10.1016/j.ebiom.2022.104294. Epub 2022 Oct 4. EBioMedicine. 2022. PMID: 36206622 Free PMC article.
-
One-Year Sustained Cellular and Humoral Immunities in Coronavirus Disease 2019 (COVID-19) Convalescents.Clin Infect Dis. 2022 Aug 24;75(1):e1072-e1081. doi: 10.1093/cid/ciab884. Clin Infect Dis. 2022. PMID: 34609506 Free PMC article.
-
How to interpret and use COVID-19 serology and immunology tests.Clin Microbiol Infect. 2021 Jul;27(7):981-986. doi: 10.1016/j.cmi.2021.05.001. Epub 2021 May 8. Clin Microbiol Infect. 2021. PMID: 33975005 Free PMC article. Review.
-
Enzyme-Linked Immunosorbent Assay: Types and Applications.Methods Mol Biol. 2023;2612:1-17. doi: 10.1007/978-1-0716-2903-1_1. Methods Mol Biol. 2023. PMID: 36795355 Review.
Cited by
-
Enhanced T-cell immunity and lower humoral responses following 5-dose SARS-CoV-2 vaccination in patients with inborn errors of immunity compared with healthy controls.Front Immunol. 2025 Mar 6;16:1538453. doi: 10.3389/fimmu.2025.1538453. eCollection 2025. Front Immunol. 2025. PMID: 40114918 Free PMC article.
-
Advancements in Ion Mobility-Based Diagnostics for Infectious Diseases.Proteomics. 2025 Jul;25(14):e13976. doi: 10.1002/pmic.13976. Epub 2025 Jun 11. Proteomics. 2025. PMID: 40495723 Free PMC article. Review.
-
QuantiFERON SARS-CoV-2 assay for the evaluation of cellular immunity after immunization with mRNA SARS-CoV-2 vaccines: a systematic review and meta-analysis.Immunol Res. 2024 Dec 27;73(1):25. doi: 10.1007/s12026-024-09570-w. Immunol Res. 2024. PMID: 39729138
-
A systematic review of T cell epitopes defined from the proteome of human immunodeficiency virus.Virus Res. 2025 Aug;358:199602. doi: 10.1016/j.virusres.2025.199602. Epub 2025 Jun 23. Virus Res. 2025. PMID: 40562176 Free PMC article. Review.
-
A Quantitative Detection Algorithm for Multi-Test Line Lateral Flow Immunoassay Applied in Smartphones.Sensors (Basel). 2023 Jul 14;23(14):6401. doi: 10.3390/s23146401. Sensors (Basel). 2023. PMID: 37514695 Free PMC article.
References
-
- Crowther J.R. The ELISA Guidebook. 2nd ed. Humana Press Inc.; Vienna, Austria: 2009.
-
- Ong D.S.Y., Fragkou P.C., Schweitzer V.A., Chemaly R.F., Moschopoulos C.D., Skevaki C., European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Respiratory Viruses (ESGREV) How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021;27:981–986. doi: 10.1016/j.cmi.2021.05.001. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

