Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy
- PMID: 35329846
- PMCID: PMC8951429
- DOI: 10.3390/jcm11061522
Value of CT-Textural Features and Volume-Based PET Parameters in Comparison to Serologic Markers for Response Prediction in Patients with Diffuse Large B-Cell Lymphoma Undergoing CD19-CAR-T Cell Therapy
Abstract
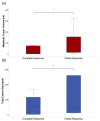
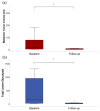
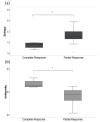
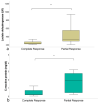
The goal of this study was to investigate the value of CT-textural features and volume-based PET parameters in comparison to serologic markers for response prediction in patients with diffuse large B-cell lymphoma (DLBCL) undergoing cluster of differentiation (CD19)-chimeric antigen receptor (CAR)-T cell therapy. We retrospectively analyzed the whole-body (WB)-metabolic tumor volume (MTV), the WB-total lesion glycolysis (TLG) and first order textural features derived from 18F-FDG-PET/CT, as well as serologic parameters (C-reactive protein [CRP] and lactate dehydrogenase [LDH], leucocytes) prior and after CAR-T cell therapy in 21 patients with DLBCL (57.7 ± 14.7 year; 7 female). Interleukin 6 (IL-6) and IL-2 receptor peaks were monitored after treatment onset and compared with patient outcome judged by follow-up 18F-FDG-PET/CT. In 12/21 patients (57%), complete remission (CR) was observed, whereas 9/21 patients (43%) showed partial remission (PR). At baseline, WB-MTV and WB-TLG were lower in patients achieving CR (35 ± 38 mL and 319 ± 362) compared to patients achieving PR (88 ± 110 mL and 1487 ± 2254; p < 0.05). The “entropy” proved lower (1.81 ± 0.09) and “uniformity” higher (0.33 ± 0.02) in patients with CR compared to PR (2.08 ± 0.22 and 0.28 ± 0.47; p < 0.05). Patients achieving CR had lower levels of CRP, LDH and leucocytes at baseline compared to patients achieving PR (p < 0.05). In the entire cohort, WB-MTV and WB-TLG decreased after therapy onset (p < 0.01) becoming not measurable in the CR-group. Leucocytes and CRP significantly dropped after therapy (p < 0.01). The IL-6 and IL-2R peaks after therapy were lower in patients with CR compared to PR (p > 0.05). In conclusion, volume-based PET parameters derived from PET/CT and CT-textural features have the potential to predict therapy response in patients with DLBCL undergoing CAR-T cell therapy.
Keywords: chimeric antigen receptor T cells; computed tomography; diffuse-large B cell lymphoma; positron emission tomography; response assessment; texture analysis.
Conflict of interest statement
Marius Horger received institutional research funds and speaker’s honorarium from Siemens Healthineers and is a scientific advisor of Siemens Healthcare Germany. The other authors have declared that no competing interests exist.
Figures

References
-
- Rovira J., Valera A., Colomo L., Setoain X., Rodríguez S., Martínez-Trillos A., Giné E., Dlouhy I., Magnano L., Gaya A., et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann. Hematol. 2014;94:803–812. doi: 10.1007/s00277-014-2271-1. - DOI - PMC - PubMed
-
- Bishton M., Hughes S.M., Richardson F., James E., Bessell E., Sovani V., Ganatra R., Haynes A.P., McMillan A.K., Fox C.P. Delineating outcomes of patients with diffuse large b cell lymphoma using the national comprehensive cancer network-international prognostic index and positron emission tomography-defined remission status; a population-based analysis. Br. J. Haematol. 2015;172:246–254. doi: 10.1111/bjh.13831. - DOI - PubMed
-
- Gisselbrecht C., Glass B., Mounier N., Gill D.S., Linch D.C., Trneny M., Bosly A., Ketterer N., Shpilberg O., Hagberg H., et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J. Clin. Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. - DOI - PMC - PubMed
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

